Abstract
Objective
To analyze the epidemiological information of patients with traumatic spinal cord injury (SCI) and concomitant traumatic brain injury (TBI) and to suggest points to be aware of during the initial physical examination of patients with SCI.
Methods
This study was a retrospective, observational study conducted in a regional trauma center. All the records of patients diagnosed with traumatic SCI between 2016 and 2020 were reviewed. A total of 627 patients with confirmed traumatic SCI were hospitalized. A retrospective study was conducted on 363 individuals.
Results
The epidemiological data of 363 individuals were investigated. Changes in American Spinal Injury Association Impairment Scale (AIS) scores in patients with SCI were evaluated. The initial evaluation was performed on average 11 days after the injury, and a follow-up examination was performed 43 days after. Fourteen of the 24 patients identified as having AIS A and SCI with concomitant TBI in the initial evaluation showed neurologic level of injury (NLI) recovery with AIS B or more. The conversion rate in patients with SCI and concomitant TBI exceeded that reported in previous studies in individuals with SCI.
The incidence of spinal cord injury (SCI) and concomitant traumatic brain injury (TBI) varies from 25% to more than 60%, depending on the criteria used [1]. Physical, cognitive, and emotional impairments caused by TBI impede patient rehabilitation [2]. Prompt diagnosis of concomitant TBI in patients with SCI is vital for appropriate rehabilitation to manage TBI-related medical complications and maximize functional recovery [3,4]. In addition to having TBI affect the patient’s prognosis, problems such as consciousness, cognitive deficits, and agitation impact the initial neurological examination of patients with SCI. Accompanied by mechanical ventilators, sedation, and psychiatric illness also affect the initial neurological examination of patients with SCI [5]. Among them, TBI is more concerned with being underdiagnosed than other factors. The initial imaging evaluation of the brain might be normal; however, accompanying head trauma should always be considered when there are symptoms of loss of consciousness or cognitive impairment [6,7]. This study aimed to summarize the epidemiology of patients with SCI and concomitant TBI at a regional level I trauma center and suggest considerations for the initial evaluation.
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Pusan National University Hospital (IRB No. 2302-013-124). The informed consent requirement was waived due to the study’s retrospective nature.
This retrospective observational study was conducted at Pusan National University Hospital, Regional Trauma Center. All records of individuals diagnosed with traumatic-spinal cord injury (T-SCI) between 2016 and 2020 were reviewed. In total, 627 patients with confirmed T-SCI were hospitalized. A retrospective study was conducted on 363 individuals, excluding those with insufficient SCI documentation, such as the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), nontraumatic etiology, or old SCI. Patients who underwent at least two ISNCSCI evaluations were included in this study (Fig. 1). The institutional ethics review board approved this study.
The TBI diagnostic criteria by the American Congress of Rehabilitation Medicine applied [8]. Based on the degree of loss of consciousness posttraumatic amnesia (PTA), imaging findings, and neuropsychological findings [7,9-12], the TBI severity was classified as mild, moderate, or severe. The highest rating of severity received was used to define each patient’s TBI severity.
Typical areas of cognitive decline in people with TBI involve visuospatial, delayed recall, attention, and language [13]. The Montreal Cognitive Assessment (MoCA) is an excellent tool for evaluating this. The average MoCA score was 19, 18, and 13 points for patients with mild, moderate, and severe TBI, respectively (Table 1). Based on this, a Korean version of MoCA score of 19 or higher was considered mild TBI, 14–18 as moderate, and 13 or less as severe TBI.
The neurological examinations analyzed retrospectively in this study were performed only by skilled rehabilitation physicians who completed online training at the American Spinal Injury Association (ASIA) e-learning center; therefore, the physical examinations performed were reliable. After the accident, an initial assessment was quickly conducted when cooperation was possible, and a follow-up examination was carried out before discharge.
Statistical analysis were performed using R version 4.2.1 (R Foundation). An independent t-test or Wilcoxon rank-sum test was conducted for continuous variables based on normality. The chi-square test or Fisher’s exact test was used for categorical variables. Statistical significance was set at a two-tailed p-value<0.05.
Table 2 summarizes the epidemiology of 363 patients. Sex, age, neurologic level of injury (NLI), American Spinal Injury Association Impairment Scale (AIS) score, and traumatic etiology information of 363 patients with confirmed T-SCI were classified. In addition, differences in characteristics were analyzed by categorizing only the SCI patient group and the SCI with the concomitant TBI patient group.
Among 363 patients with T-SCI, 296 (81.5%) were males, and 67 (18.5%) were females. The average age was 57.1 years. C1–4 injuries accounted for 54.8% of the patients. This was followed by affected C5–8 at 20.4%, T1–12 at 14.0%, and L1–S5 at 8.0%. AIS A, B, C, and D accounted for 25.1%, 7.7%, 26.7%, and 37.2%, respectively. T-SCI etiology was classified as fall, transport, sports and leisure, assault, and other traumatic causes [14]. Among 363 patients, fall was the most common cause (205, 56.5%), followed by transport (113, 31.1%). When calculating the p-value, the unknown initial NLI or AIS scores were regarded as missing data. Supplementary Table S1 describes the epidemiological information reviewed above by subdividing patients with SCI and concomitant TBI according to the TBI severity criteria.
Table 3 shows the changes in the AIS changes in patients with SCI only and those in SCI patients with concomitant TBI (SCI+TBI). AIS conversion was examined by classifying the SCI-only patient groups and SCI+TBI. In addition, the subgroups were divided according to the TBI severity. In total, 318 patients had ISNCSCI follow-up records. The initial evaluation was performed 11 days (average) after the injury, and a follow-up was performed 43 days after.
In this study, ISNCSCI was performed in some patients, even in those with severe TBI. Since the initial evaluation of the injury determined TBI severity, the Glasgow Coma Scale (GCS) score was 3–8 during the trauma emergency room admission; however, the level of consciousness recovered subsequently, and ISNCSCI could be performed.
Fourteen of the 24 patients diagnosed with SCI+TBI and identified as AIS A in the initial evaluation showed neurologic recovery compared with AIS B or higher (Table 3). In contrast, in those with T-SCI without head injury, neurological recovery was confirmed in only 16 of 62 patients initially evaluated as AIS A (Fig. 2). In only patients with SCI, the conversion rate was 0.258 (95% confidence interval [CI], 0.149–0.367), whereas, in SCI+TBI, it was 0.583 (95% CI, 0.386–0.781). The conversion rate was statistically significant (p=0.010) between the SCI and the SCI+TBI groups.
Supplementary Table S2 summarizes the sex, age, TBI severity, NLI, and AIS conversion of patients with SCI+TBI whose AIS conversion was confirmed from initial AIS A to AIS B or higher.
This study investigated the epidemiology of patients with SCI alone and those with SCI+TBI. In Korea, regional trauma centers were opened in 2014, and 16 centers are currently operational. This was a detailed retrospective analysis of T-SCI in a single institution after establishing a systematic regional trauma center in Korea. There is a lack of data worldwide, and this is the first study in Korea to investigate patients with SCI+TBI. A significant difference in the AIS conversion rate between the two groups was found.
Since there is a lack of consensus on evaluating patients with SCI+TBI, this study aimed to suggest considerations for initial evaluation, including ISNCSCI.
Among 363 patients with T-SCI, 296 (81.5%) were males, similar to the global 4:1 male-to-female ratio of T-SCI [15]. Similarly, according to spine epidemiological studies conducted in Korea, the ratio was 3.6:1 in the 2010s [16].
According to Asian studies, males were at a higher risk of T-SCI; the sex ratio ranged from 0.99:1 in Taiwan to 13.5:1 in India [17,18]. This inconsistency may be due to socioeconomic status and cultural background differences. Males are more likely to participate in trauma-related physical activities [19].
The average age was 57.1 years, higher than the average age of T-SCI patients in the USA according to the 2021 National Spinal Cord Injury Statistical Center (NSCISC) annual report (42.2 years) [20]. Asian studies reported a range of 26.8–56.6 years [19]. A previous Korean study showed that the mean age at the time of injury increased from 32.4 years in the 1990s to 47.1 years in the 2010s [16].
This study’s proportion of older adults aged >reached 48.2%. The increasing proportion of older adults will cause changes in epidemiology, such as changes in traumatic etiology.
Considering this study’s initial NLI, affected C1–4 accounted for 54.8% of all patients with SCI, followed by affected C5–8 (20.4%). Cervical-level injuries accounted for 75.2% of the patients. This was followed by affected T1–12 at 14.0% and L1–S5 at 8.0%. In Korea, cervical-level injury accounted for 57.2% of all T-SCIs in the 2010s, of which NLI C4–6 accounted for the largest proportion over 30 years [16]. The proportion of cervical injuries was much higher than that in the current statistics. The higher-level cervical cord injuries above the meaning NLI C1–4 level accounted for 54.8%. This may be related to the characteristics of the regional trauma centers where patients with severe T-SCI visit.
Among the 74 patients with SCI+TBI, the affected C1–4 accounted for 43.2%, C5–8 for 18.9%, thoracic level for 20.3%, and lumbar level for 4.1%. There was no statistically significant difference in the initial NLI between the only SCI and the SCI+TBI groups. Macciocchi et al. [10] reported that the TBI co-occurrence rate is 70%–77% when the NLI is C1–4, 59%–67% for C5–8, and 11%–59% for levels below T1; therefore, higher-level cervical cord injury was shown to be common in patients with T-SCI and concomitant TBI [21]. Contrary to previous studies, the cervical-level injury rate in patients with SCI+TBI in this study was lower than that in patients with SCI alone. This may be due to the high severity of the patient group with cervical SCI+TBI who visited the regional trauma center. There may have been selection bias since ISNCSCI could not be performed for reasons such as death, critically ill medical condition, and persistent unconsciousness. In addition, the possibility of a certain number of undiagnosed TBIs due to another urgent trauma issue is considered cautiously.
When divided based on this study’s initial AIS, AIS A, B, C, and D accounted for 25.1%, 7.7%, 26.7%, and 37.2%, respectively. In existing USA statistics, AIS A, B, C, and D account for 41.9%, 10.7%, 12.4%, and 29.4% of the cases, respectively [20]. It is characteristic that the ratio of AIS A is minute, and that of AIS C is larger than previously known values. According to the 2021 NSCISC annual report, gunshot wounds account for 15.3% of all SCI etiologies, and there are few other penetrating injuries, such as stab wounds, in other countries [20,22,23]. However, in Korea, most injuries involve blunt trauma since penetrating injuries are infrequent and personal gun possession is prohibited under Korean law. Hence, it was estimated that the rate of AIS A was lower than that reported in previous studies in other Western countries or registry collection results. In addition, one of the primary etiologies of SCI is transport, and Korea’s highest seat belt-wearing rate may have contributed to this [23].
The rate of AIS A was relatively high in patients with double injuries. AIS A accounted for 22.8% of patients with SCI alone and 33.8% with double injuries. Hagen et al. [6] reported that completeness of T-SCI was strongly associated with clinical TBI. The complete injury rate among 179 individuals with SCI without TBI was only 34.6%. However, the complete injury rate reached 78.9% in individuals with SCI+severe TBI. The completeness of the T-SCI indicates high-energy trauma with an increased risk of concomitant TBI. This study was also consistent with previous studies.
Globally, transport is the most common cause of SCI, followed by falls [15]. In a Korean study, transport-based SCI decreased from 65% of all injuries in 1990–1999 to 41.9% in 2010–2019, while fall-based SCI increased from 24.9% in 1990–1999 to 46.3% in 2010–2019 [16]. Falls were the most common cause of injury in the >60 age group, resulting in 59.1% T-SCI in the 2010s [16].
In this study, among 363 patients, fall was the most common cause (205, 56.5%), followed by transport (113, 31.1%). This is presumed to be related to the high proportion of older adults, and many cases have occurred due to slipping [24]. Older adults are vulnerable to falls due to deterioration of physical functions, including balance function, musculoskeletal system, visual perception, and cognitive function problems [25]. Besides, degeneration of various components of the vertebra is common in the elderly population. Spinal degenerative changes such as ossification of the posterior longitudinal ligament, disc disease, stenosis, and spondylolisthesis cause a higher risk of suffering SCI following a fall or another traumatic event in older adults [26]. Also, attempts to socially reduce traffic accidents, such as wearing seat belts and regulating the speed limit, could explain this change in etiology [27,28]. Other traumatic causes identified in this study included falling objects and industrial accidents, which accounted for 7.7% of the total, with 28 cases.
Among the 113 patients with transport in this study, 32 (28.3%) had TBI. Among the 205 patients with falls, 41 (20.0%) had TBI. There was no significant difference in TBI comorbidities between the two major etiology groups.
Most importantly, as shown in Table 3, the initial AIS was A in patients with only SCI; however, the conversion rate to AIS B, C, or D in the follow-up examination was 25.8%. In contrast, in patients with SCI+TBI, AIS conversion was 58.3% (p-value 0.010). In a previous study, 20%–30% of individuals with AIS A SCI at baseline examination (within 30 days of injury) converted to an incomplete status [29-31]. However, existing studies regarding AIS conversion do not accurately identify whether TBI accompanies SCI. Besides, neurological conversion is rare after complete paraplegia (~15%–20%) relative to tetraplegia [29]. The higher neurological conversion in patients with tetraplegia than in those with paraplegia in a previous study is thought to be partly influenced by differences in the TBI frequency.
This was a single-center evaluation; however, the AIS conversion rate in SCI patients with TBI was significantly higher than that in patients with SCI alone. The patient might have been initially evaluated as AIS A due to decreased cognition and cooperation, resulting in high AIS conversion.
Supplementary Table S2 shows the patients who had AIS A at the initial evaluation but deviated from AIS A at the follow-up evaluation. Even if not accompanied by moderate or severe TBI, four patients with SCI concomitant with mild TBI were converted to AIS B or C. The two persons who were accompanied by mild TBI but converted from AIS A to C were intubated during evaluation or lightly sedated with a score of -1 on the Richmond Agitation-Sedation Scale (RASS).
In the intensive care unit, the degree of sedation and agitation was objectively indicated using the RASS [32]. The RASS score was classified from -5 to +5 points, and patients with a score between -1 and +1 points were considered eligible for reliable physical examination in this study. Since complete injury judgment is crucial in the initial evaluation, it was possible to identify deep anal pressure and voluntary anal contraction between RASS -1 and +1. In addition to accompanying TBI, other factors that may affect physical examination findings, such as endotracheal intubation, sedation, and delirium, should be closely considered. The initial AIS assessment could be incorrect owing to the influence of concomitant TBI lesions or decreased consciousness/cognitive state due to the factors mentioned above.
There are no clear standards for the arousal, awareness, or cooperation required to implement ISNCSCI. Even if ISNCSCI evaluation is possible, it is desirable to record the GCS score during evaluation and include cognitive evaluation results such as the mini-mental state examination (MMSE), MoCA, RASS score, and Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) indicating arousal, awareness, and cooperation (Supplementary Table S3). Moreover, interpreting ISNCSCI will be easier if the examiner records the presence of severe pain that may limit the physical examination, endotracheal intubation status, and level of cognitive decline due to the underlying disease.
ISNCSCI is known to show discrepancies among experienced examiners [33]. The evaluation and scoring are challenging. In the SCI+TBI group, there were many restrictions on the implementation of reliable ISNCSCI due to the reasons discussed above. Therefore, commenting regarding consciousness and cognition during evaluation is crucial.
Furthermore, patients with an initial decline in consciousness and cognition need close follow-up since the possibility of neurological alterations in ISNCSCI may be high. Regarding the minimum requirements to properly perform ISNCSCI evaluation, further prospective studies are required on the arousal, awareness, and cooperation criteria.
In this study, serial cognitive function tests were rarely performed in patients with AIS conversion. There are limitations to performing serial cognitive function tests during the acute treatment period in patients with trauma. A series of cognitive evaluation tests are recommended in patients initially evaluated as having AIS A but whose neurologic recovery was beyond AIS B in the follow-up evaluation. Suppose AIS conversion is confirmed along with cognitive improvement in a series of evaluations. In that case, it can be inferred that the inaccurate ISNCSCI assessment is due to cognitive decline in the early phase of the injury.
The study has certain limitations. Since it was a retrospective study, the timing of initial and follow-up evaluations was inconsistent. The small sample size was not representative of the characteristics of Korea. The data were limited to a single university hospital. Considering the selection bias of a single institution, building a registry that includes the evaluation items that were limited in this study is necessary.
In conclusion, the study presents the epidemiology of SCI with concomitant TBI, lacking in research worldwide, and is the first study in Korea. Identifying the coexistence of TBI through the current national SCI registry is challenging. Hence, this is a relevant study. Establishing a global SCI patient registry and checking the medical records necessary for evaluation and follow-up is needed. In particular, when ISNCSCI is accompanied by TBI, a specific global consensus on the evaluation must be reached through further studies. The key issue is when a reliable physical examination can be performed.
Notes
This work was supported by a clinical research grant from Pusan National University Hospital in 2023.
Conceptualization: Yang TW, Shin YB, Kim SH. Methodology: Yang TW, Shin YB, Kim SH. Formal analysis: Yang TW. Funding acquisition: Huh S, Kim SH. Project administration: Yang TW, Jang MH, Shin YB, Kim SH. Visualization: Yang TW, Yoo DH. Writing – original draft: Yang TW, Kim SH. Writing – review and editing: Huh S, Jang MH, Shin YB, Kim SH. Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.5535/arm.23054.
Summary of current evidence of neurodevelopmental assessment tool
Supplementary Table S1.
Study characteristics in SCI with TBI patients
Supplementary Table S2.
NLI and AIS conversion of initial AIS A in SCI with concomitant TBI
Supplementary Table S3.
Consideration points about arousal and awareness in the ISNCSCI worksheet
REFERENCES
1. Budisin B, Bradbury CC, Sharma B, Hitzig SL, Mikulis D, Craven C, et al. Traumatic brain injury in spinal cord injury: frequency and risk factors. J Head Trauma Rehabil. 2016; 31:E33–42.

2. Bradbury CL, Wodchis WP, Mikulis DJ, Pano EG, Hitzig SL, McGillivray CF, et al. Traumatic brain injury in patients with traumatic spinal cord injury: clinical and economic consequences. Arch Phys Med Rehabil. 2008; 89(12 Suppl):S77–84.

3. Garlanger KL, Beck LA, Cheville AL. Functional outcomes in patients with co-occurring traumatic brain injury and spinal cord injury from an inpatient rehabilitation facility’s perspective. J Spinal Cord Med. 2018; 41:718–30.

4. Macciocchi SN, Bowman B, Coker J, Apple D, Leslie D. Effect of co-morbid traumatic brain injury on functional outcome of persons with spinal cord injuries. Am J Phys Med Rehabil. 2004; 83:22–6.

5. Burns AS, Lee BS, Ditunno JF Jr, Tessler A. Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J Neurotrauma. 2003; 20:477–82.

6. Hagen EM, Eide GE, Rekand T, Gilhus NE, Gronning M. Traumatic spinal cord injury and concomitant brain injury: a cohort study. Acta Neurol Scand Suppl. 2010; (190):51–7.

7. Tolonen A, Turkka J, Salonen O, Ahoniemi E, Alaranta H. Traumatic brain injury is under-diagnosed in patients with spinal cord injury. J Rehabil Med. 2007; 39:622–6.

8. Ruff RM, Iverson GL, Barth JT, Bush SS, Broshek DK; NAN Policy and Planning Committee. Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol. 2009; 24:3–10.

9. de Guise E, Alturki AY, LeBlanc J, Champoux MC, Couturier C, Lamoureux J, et al. The Montreal Cognitive Assessment in persons with traumatic brain injury. Appl Neuropsychol Adult. 2014; 21:128–35.

10. Macciocchi S, Seel RT, Thompson N, Byams R, Bowman B. Spinal cord injury and co-occurring traumatic brain injury: assessment and incidence. Arch Phys Med Rehabil. 2008; 89:1350–7.

11. de Guise E, Leblanc J, Champoux MC, Couturier C, Alturki AY, Lamoureux J, et al. The mini-mental state examination and the Montreal Cognitive Assessment after traumatic brain injury: an early predictive study. Brain Inj. 2013; 27:1428–34.

12. Sun H, Luo C, Chen X, Tao L. Assessment of cognitive dysfunction in traumatic brain injury patients: a review. Forensic Sci Res. 2017; 2:174–9.

13. Miotto EC, Cinalli FZ, Serrao VT, Benute GG, Lucia MC, Scaff M. Cognitive deficits in patients with mild to moderate traumatic brain injury. Arq Neuropsiquiatr. 2010; 68:862–8.

14. Biering-Sørensen F, Charlifue S, Chen Y, New PW, Noonan V, Post MWM, et al. International Spinal Cord Injury Core Data Set (version 3.0)-including standardization of reporting. Spinal Cord. 2023; 61:65–8.
15. Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014; 6:309–31.
16. Lee BS, Kim O, Ham D. Epidemiological changes in traumatic spinal cord injuries for the last 30 years (1990-2019) in South Korea. Spinal Cord. 2022; 60:612–7.

17. Yang NP, Deng CY, Lee YH, Lin CH, Kao CH, Chou P. The incidence and characterisation of hospitalised acute spinal trauma in Taiwan--a population-based study. Injury. 2008; 39:443–50.

18. Chacko V, Joseph B, Mohanty SP, Jacob T. Management of spinal cord injury in a general hospital in rural India. Paraplegia. 1986; 24:330–5.

19. Ning GZ, Wu Q, Li YL, Feng SQ. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J Spinal Cord Med. 2012; 35:229–39.

20. National Spinal Cord Injury Statistical Center (NSCISC). The 2021 annual statistical report complete public version for the Spinal Cord Injury Model Systems. University of Alabama at Birmingham;2022. p. 1–161.
21. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002; 96(3 Suppl):285–91.

22. Barbetta DC, Smanioto TR, Poletto MF, Ferreira R, Lopes A, Casaro FM, et al. Spinal cord injury epidemiological profile in the Sarah Network of Rehabilitation Hospitals-a Brazilian population sample. Spinal Cord Ser Cases. 2018; 4:32.

23. International Transport Forum. Road safety annual report 2020. International Transport Forum;2020. p. 1–64.
24. Kim HS, Lim KB, Kim J, Kang J, Lee H, Lee SW, et al. Epidemiology of spinal cord injury: changes to its cause amid aging population, a single center study. Ann Rehabil Med. 2021; 45:7–15.

25. Harper A, Wilkinson I. Falls in older adults: causes, assessment and management. Medicine. 2021; 49:32–7.

26. Rhee JM, Shamji MF, Erwin WM, Bransford RJ, Yoon ST, Smith JS, et al. Nonoperative management of cervical myelopathy: a systematic review. Spine (Phila Pa 1976). 2013; 38(22 Suppl 1):S55–67.
27. Chamberlain JD, Deriaz O, Hund-Georgiadis M, Meier S, Scheel-Sailer A, Schubert M, et al. Epidemiology and contemporary risk profile of traumatic spinal cord injury in Switzerland. Inj Epidemiol. 2015; 2:28.

28. Knútsdóttir S, Thórisdóttir H, Sigvaldason K, Jónsson H Jr, Björnsson A, Ingvarsson P. Epidemiology of traumatic spinal cord injuries in Iceland from 1975 to 2009. Spinal Cord. 2012; 50:123–6.

29. Kirshblum S, Snider B, Eren F, Guest J. Characterizing natural recovery after traumatic spinal cord injury. J Neurotrauma. 2021; 38:1267–84.

30. Khorasanizadeh M, Yousefifard M, Eskian M, Lu Y, Chalangari M, Harrop JS, et al. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine. 2019; 30:683–99.

31. Spiess MR, Müller RM, Rupp R, Schuld C; EM-SCI Study Group, van Hedel HJ. Conversion in ASIA impairment scale during the first year after traumatic spinal cord injury. J Neurotrauma. 2009; 26:2027–36.

Fig. 1.
Flow diagram of finding patients with traumatic spinal cord injury (T-SCI) and concomitant traumatic brain injury (TBI).
Fig. 2.
Changes in American Spinal Injury Association Impairment Scale (AIS) scores (A) only spinal cord injury (SCI), (B) SCI+traumatic brain injury (TBI), (C) mild TBI, (D) moderate TBI, (E) severe TBI.
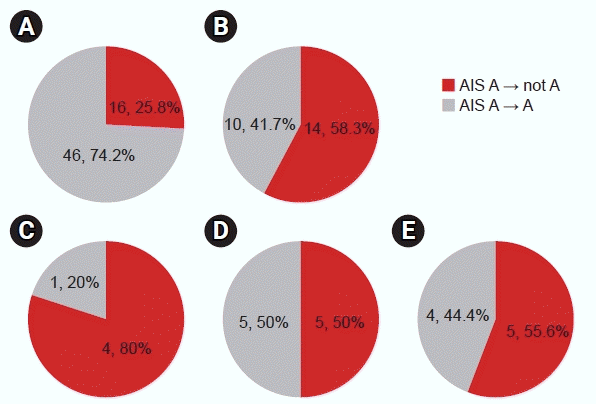
Table 1.
Diagnostic criteria for the classification of TBI severity
Table 2.
Study characteristics
Table 3.
Change of AIS




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download