Abstract
Background
This study investigated the risk of cause-specific mortality according to glucose tolerance status in elderly South Koreans.
Methods
A total of 1,292,264 individuals aged ≥65 years who received health examinations in 2009 were identified from the National Health Information Database. Participants were classified as normal glucose tolerance, impaired fasting glucose, newly-diagnosed diabetes, early diabetes (oral hypoglycemic agents ≤2), or advanced diabetes (oral hypoglycemic agents ≥3 or insulin). The risk of system-specific and disease-specific deaths was estimated using multivariate Cox proportional hazards analysis.
Results
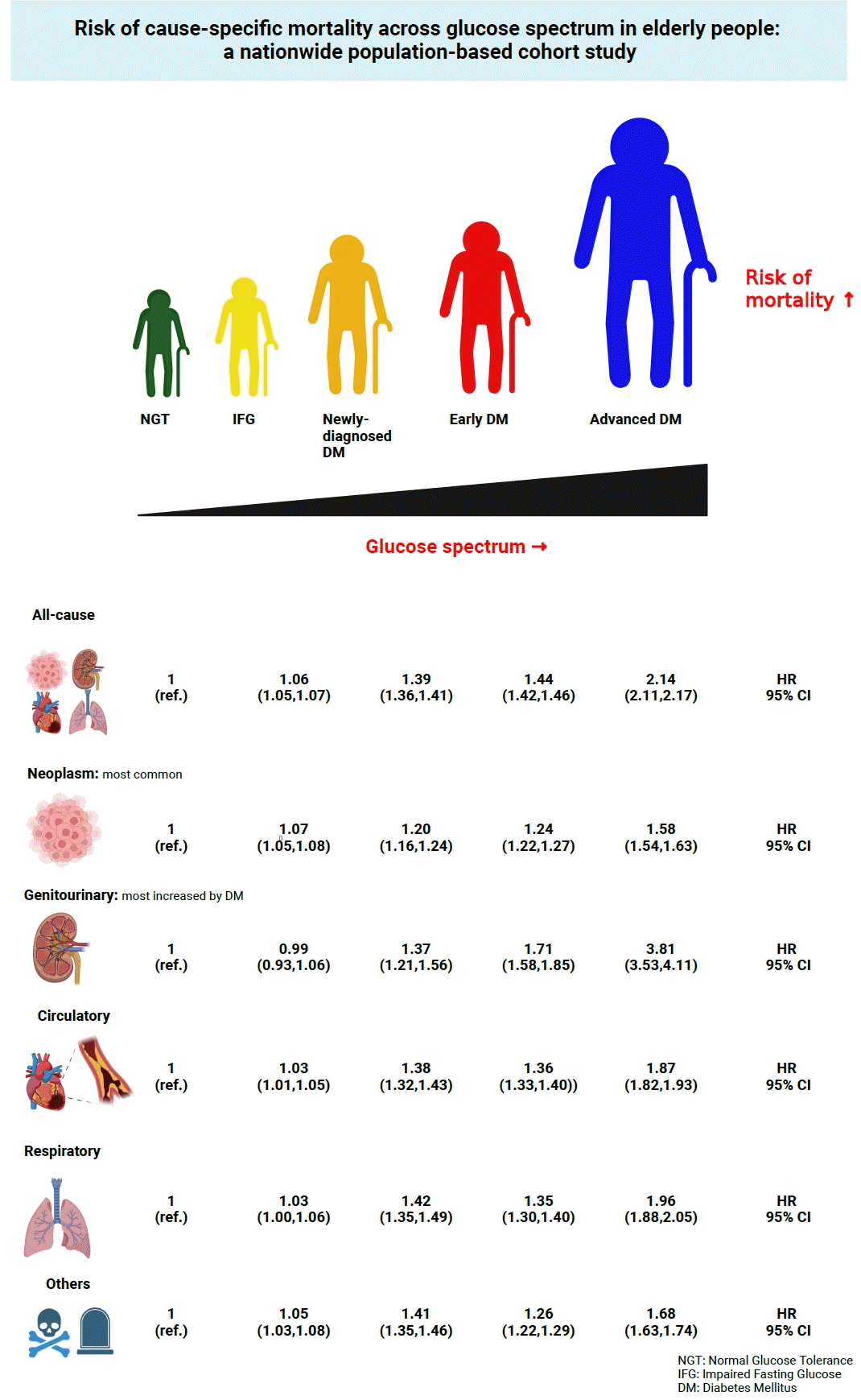
During a median follow-up of 8.41 years, 257,356 deaths were recorded. Diabetes was associated with significantly higher risk of all-cause mortality (hazard ratio [HR], 1.58; 95% confidence interval [CI], 1.57 to 1.60); death due to circulatory (HR, 1.49; 95% CI, 1.46 to 1.52), respiratory (HR, 1.51; 95% CI, 1.47 to 1.55), and genitourinary systems (HR, 2.22; 95% CI, 2.10 to 2.35); and neoplasms (HR, 1.30; 95% CI, 1.28 to 1.32). Diabetes was also associated with a significantly higher risk of death due to ischemic heart disease (HR, 1.70; 95% CI, 1.63 to 1.76), cerebrovascular disease (HR, 1.46; 95% CI, 1.41 to 1.50), pneumonia (HR, 1.69; 95% CI, 1.63 to 1.76), and acute or chronic kidney disease (HR, 2.23; 95% CI, 2.09 to 2.38). There was a stepwise increase in the risk of death across the glucose spectrum (P for trend <0.0001). Stroke, heart failure, or chronic kidney disease increased the risk of all-cause mortality at every stage of glucose intolerance.
Diabetes mellitus (DM) is a highly prevalent chronic condition among the elderly. Approximately 20% to 30% of the elderly population is estimated to have diabetes, although the numbers vary among countries [1-3]. Elderly patients with diabetes commonly have other medical conditions (e.g., cardiovascular disease, chronic kidney disease, or heart failure [HF]) and are at an increased risk of mortality, which may impose a substantial socioeconomic burden on society [4-6]. Furthermore, accumulating evidence indicates that mortality trends in patients with diabetes are changing [7-9]. However, the characteristics of death in the elderly population with diabetes are poorly understood. Even in the elderly population with diabetes, each individual has a different risk of mortality. Therefore, categorizing the elderly population with diabetes according to their risk of mortality and stratifying their cause-specific risk of death are important for efficiently managing limited public health resources. In this study, we describe the cause-specific risk of death in the South Korean elderly population and suggest a novel method to categorize the elderly population with respect to the risk of mortality.
This study was based on the Korean National Health Information Database (NHID), which combines data from the National Health Insurance Service (NHIS) collected for claims and reimbursement of healthcare services and general health examinations [10]. The NHIS is a single public health insurance provider that covers 97% of the South Korean population. It offers health insurance, administers subscriptions for insured individuals and their dependents, collects payments, and establishes payment schedules for medical bills [11]. Every South Korean aged 20 years or older is mandated to undergo public health screening at least every 2 years. Customized claims data from the NHID were obtained after de-identification. Details of the variables included in the NHID and health examinations are described elsewhere [12]. Data from the National Death Registry of Statistics Korea were merged to further integrate information regarding the cause and number of death events. The study protocol was reviewed and approved by the Institutional Review Board of Seoul St. Mary’s Hospital, Catholic University of Korea (approval number KC23ZISI0013). The requirement for informed consent was waived, because only de-identified data were used.
We identified 10,585,844 adults who underwent the national health examination in 2009. We excluded people younger than 65 years (n=9,193,743), those with missing data (n=84,985), and those who died within 1 year of follow-up (n=14,852). Finally, 1,292,264 participants were enrolled and analyzed. This was a longitudinal retrospective observational study with a median follow-up period of 8.41 years (interquartile range, 8.06 to 8.72).
The study participants were categorized into five groups according to the glucose spectrum: normal glucose tolerance (NGT), impaired fasting glucose (IFG), newly diagnosed DM, early DM, and advanced DM. NGT was defined as no history of diabetes (no disease code recorded or history of antidiabetic medication prescription) and a baseline fasting glucose level <100 mg/dL. IFG was defined as no history of diabetes and a baseline fasting glucose level ≥100 and <126 mg/dL. Newly-diagnosed DM was defined as no history of diabetes but with a fasting glucose level ≥126 mg/dL at health examination [13]. Early DM was defined as having a history of diabetes and being prescribed zero to two classes of antidiabetic medication. Advanced DM was defined as a history of diabetes and using three or more classes of antidiabetic medication or insulin treatment.
Body mass index (BMI) was calculated as weight divided by the square of height (kg/m2). Blood glucose, total cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels were measured after an overnight fast. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula: 186×(serum creatinine)–1.154×age–0.203×0.742 (if female), and chronic kidney disease was defined as eGFR <60 mL/min/1.73 m2. Information regarding smoking history and alcohol consumption was obtained using a self-reported questionnaire. Drinking >30 g/day of alcohol was considered heavy consumption. Regular exercise was defined as performing >30 minutes of moderate-intensity activity at least five times a week or >20 minutes of vigorous-intensity exercise at least three times a week [14]. Hypertension was defined as systolic blood pressure (BP) ≥140 mm Hg, diastolic BP ≥90 mm Hg, or at least one prescription of antihypertensive agents per year under International Classification of Disease, 10th revision (ICD-10) codes I10–I11. Dyslipidemia was defined as a total cholesterol level ≥240 mg/dL or at least one prescription of an antihyperlipidemic agent under ICD-10 code E78. History of ischemic heart disease (I20–I25), stroke (I63, I64), HF (I50), and cancer (C00–C97) was identified based on ICD-10 codes.
Death was the primary outcome of the study. We collected all-cause, system-specific, and disease-specific mortality data of enrolled participants. The system and disease-specific causes of death were based on ICD-10 codes. System-specific death events were classified as circulatory system (code I), respiratory system (code J), genitourinary system (code N), neoplasm (code C), and others. Disease-specific death events were categorized as ischemic heart disease (I20–I25), cerebrovascular disease (I60–I69), pneumonia (J12–J18), and acute kidney failure and chronic kidney disease (N17–N19).
Baseline characteristics according to the glucose spectrum groups are described as the mean±standard deviation, median (interquartile range), or number (%). The incidence rate of the outcomes was calculated by dividing the total number of events by the follow-up period (person-years). A multivariate-adjusted Cox regression analysis was performed to estimate the risk (hazard ratio [HR]) of mortality according to the presence of DM or glucose spectrum. The Schoenfeld residual test, with a logarithm of the cumulative hazard functions based on Kaplan–Meier estimates, was used to evaluate the proportional-hazards assumption. There was no significant departure from the proportionality to hazards over time. Model 1 was adjusted for age and sex, and model 2 was further adjusted for smoking, heavy alcohol consumption, regular exercise, BMI, hypertension, and dyslipidemia. Sensitivity analysis was performed after excluding patients with underlying malignancies. Subgroup analyses were conducted based on age (<75 or ≥75 years), sex, or underlying diseases (stroke, HF, or chronic kidney disease), and the potential effects modification was evaluated through stratified analysis and interaction testing using a likelihood-ratio test. Statistical significance was set at P<0.05. SAS version 9.4 was used for all statistical analyses (SAS Institute, Cary, NC, USA).
Among the 1,292,264 participants, 260,301 (20.1%) had diabetes at baseline. The enrolled participants were categorized into five groups according to their glucose spectrum: NGT (n=693,831, 53.7%), IFG (n=338,132, 26.2%), newly diagnosed DM (n=50,618, 3.9%), early DM (n=132,396, 10.2%), and advanced DM (n=77,314, 6.0%). The baseline characteristics of the participants across the glucose spectrum are shown in Table 1. Their mean age was approximately 71 years. The degree of obesity and abdominal obesity was higher in individuals with diabetes than in normoglycemic participants. BP, fasting glucose, lipid profiles, and rates of current smoking and heavy alcohol consumption were the highest in people with newly diagnosed DM. The prevalence of comorbidities, such as hypertension, dyslipidemia, chronic kidney disease, ischemic heart disease, stroke, HF, and cancer, increased according to the severity of glucose intolerance (P for trend <0.0001 for all variables).
During the follow-up period, 257,356 death events occurred. Overall, elderly people with diabetes were at an increased risk of mortality compared with the elderly population without diabetes (incidence rate 34.7 vs. 23.5 per 1,000 person-years; HR, 1.58; 95% confidence interval [CI], 1.57 to 1.60). When cause-specific mortality was analyzed, people with diabetes had higher HRs of mortality in every aspect of system categories (circulatory system [HR, 1.49; 95% CI, 1.46 to 1.52], respiratory system [HR, 1.51; 95% CI, 1.47 to 1.55], genitourinary system [HR, 2.22; 95% CI, 2.10 to 2.35], neoplasms [HR, 1.30; 95% CI, 1.28 to 1.32], and others [HR, 1.38; 95% CI, 1.35 to 1.41]), with highest hazards for genitourinary system. The incidence rate of death in patients with diabetes was the highest for neoplasms (9.9 per 1,000 person-years) among the various system categories. We further explored the differences in the mortality risk across the glucose spectrum. Indeed, the risk of mortality increased stepwise according to the severity of glucose intolerance in all-cause and system-specific deaths (all P<0.0001). Within the glucose spectrum, the risk of mortality markedly increased from early to advanced DM for all causes of death (Table 2). Although neoplasms were the most common cause of death in the elderly population, the contribution of diabetes to the risk of death caused by neoplasms was relatively low. Because cancer per se is an important factor for mortality in patients with cancer, we further analyzed the cause-specific mortality trend after excluding participants with preexisting cancers at baseline. However, neoplasms were the most common cause of death in elderly people with diabetes (incidence rate 8.8 per 1,000 person-years). The multivariate adjusted HR for mortality was highest by genitourinary system (HR, 2.22; 95% CI, 2.10 to 2.35), and the risk of mortality caused by every system step-wisely increased according to glucose spectrum (Supplemental Table S1). We also examined the proportions of cause of death within each category of the glucose spectrum. Neoplasm was consistently the most common cause of death across all glucose spectrum categories, followed by the circulatory system, respiratory system, and genitourinary system. The relative proportion of deaths caused by the circulatory system and genitourinary system tended to increase according to the glucose spectrum (Supplemental Fig. S1).
To further describe the characteristics of death in the elderly population, we analyzed the mortality risk of representative diseases across the glucose spectrum. As expected, the presence of diabetes consistently increased the risk of mortality caused by ischemic heart disease (HR, 1.70; 95% CI, 1.63 to 1.76), cerebrovascular disease (HR, 1.46; 95% CI, 1.41 to 1.50), pneumonia (HR, 1.69; 95% CI, 1.63 to 1.76), and acute kidney failure and chronic kidney disease (HR, 2.23; 95% CI, 2.09 to 2.38). The increased risk of mortality due to diabetes was most prominent among deaths caused by acute kidney failure and chronic kidney disease. Notably, the risk of mortality increased stepwise across the glucose spectrum for every cause (P for trend <0.0001). Within the glucose spectrum, the risk of mortality increased most markedly from early to advanced DM for every cause of death. The multivariate adjusted HR for death caused by acute kidney failure and chronic kidney disease was 3.96 (95% CI, 3.63 to 4.32) for advanced DM compared with NGT (Table 3).
In the elderly population with diabetes, the mortality rate is known to markedly increase after the age of 75 years [15]. Based on this knowledge, we subcategorized the participants into those aged below or over 75 years and analyzed their cause-specific mortality risk. Both age subgroups exhibited similar trends in cause-specific mortality risk in the glucose spectrum (Fig. 1). Mortality risk gradually increased across the glucose spectrum in all-cause mortality and death caused by circulatory, respiratory, and genitourinary systems; neoplasms; and others (all P for trend <0.0001). Importantly, the increase in mortality risk across the glucose spectrum was more pronounced in people aged below 75 years than those aged at or over 75 years for all causes of death (P for interaction <0.0001 except for neoplasms which was P=0.0058). The HR of mortality in participants aged <75 years was highest in the genitourinary system (HR, 4.77; 95% CI, 4.33 to 5.25). The patterns of mortality risk according to glucose spectrum were also significantly different between men and women in terms of all-cause mortality and death caused by the circulatory system, respiratory system, and others (Supplemental Fig. S2).
Elderly people commonly have serious medical conditions. Chronic kidney disease, HF, and stroke are commonly associated diseases in patients with diabetes [16,17]. We further analyzed whether the presence of these comorbidities changed the risk of mortality in the elderly population according to the glucose spectrum. The risk of mortality increased stepwise across the glucose spectrum regardless of the presence of an underlying disease. However, when participants in the same glucose spectrum were compared, the risk of all-cause mortality was consistently higher in those with comorbidities than in those without comorbidities (Fig. 2). In general, subjects with early DM and comorbidities had a similar risk of all-cause mortality as those with advanced DM and no comorbidities.
In this study, we characterized the cause-specific mortality risk in the elderly population along the glucose spectrum. We subcategorized the elderly population into normoglycemia, IFG, newly diagnosed DM, early DM, and advanced DM groups, and demonstrated that the risk of mortality increased stepwise across the glucose spectrum. This trend was consistently observed regardless of the cause of death, age, sex, and underlying diseases (chronic kidney disease, HF, and stroke). Neoplasms were the most common cause of death in the elderly population, whereas the genitourinary system was the cause of death most influenced by the presence of diabetes.
Several studies have attempted to stratify the risk of mortality in the elderly population [12,18-21]. However, these studies often relied on variables that are frequently omitted in real-world clinical settings. In our study, we aimed to classify subjects based on the diabetes spectrum by using easily obtainable variables such as fasting glucose levels, diabetes diagnosis, the number of oral hypoglycemic agents used, and insulin usage. Our classification method did not incorporate information on the presence of diabetes-related complications, which could potentially influence the risk of mortality. Therefore, our study enabled the stratification of cause-specific mortality irrespective of the underlying disease status. We propose that our classification method adequately represents the disease severity using readily available variables.
Our data provide valuable insights for health administrators regarding public resource allocation. We found that the risk of mortality was consistently increased in the IFG group, with a HR of 1.06 and 95% CI of 1.05 to 1.07, which aligns with a recent report [22]. Approximately 50% of the elderly population falls under the IFG category [2]. Consequently, greater efforts should be dedicated to promoting lifestyle modification in the elderly population with IFG, particularly in primary care settings. Furthermore, the mortality risk showed a modest increase in early DM (HR, 1.44; 95% CI, 1.42 to 1.46) and newly diagnosed DM (HR, 1.39; 95% CI, 1.36 to 1.41). Therefore, comparable attention should be given to elderly individuals with diabetes, starting from the time of DM diagnosis until they require treatment with three or more classes of antidiabetic medications or insulin. In the context of the glucose spectrum, the risk of mortality exhibited a substantial increase from early to advanced DM, contributing to deaths across various body systems. Notably, the risk of mortality in advanced DM was higher among individuals aged 65 to 75 years than those aged over 75 years. Consequently, elderly patients with diabetes who use three or more classes of oral hypoglycemic agents or receive insulin treatment require increased medical care and attention.
Neoplasms were the most common cause of death in the elderly population, and their relative risk increased with the glucose spectrum. This result is consistent with those of previous studies performed in the general diabetes population in the United Kingdom and Australia [7,23]. Cardiovascular disease was the most common cause of death in patients with diabetes owing to its high incidence and fatality rate. Improvements in coronary and cerebral revascularization techniques and medical care to control cardiovascular disease-associated risk factors (BP, glucose, and lipids) may have contributed to this change in mortality trends [24,25]. The association between hyperglycemia and the development, progression, and survival of cancer has been studied extensively [26,27]. Therefore, more resources should be provided for cancer screening and management in patients with advanced DM.
Genitourinary system disease, more specifically ‘acute kidney failure and chronic kidney disease,’ was the cause of death for which the risk was most increased by the presence of diabetes (HR, 2.23; 95% CI, 2.09 to 2.38). In particular, for those with advanced DM, the HR of death reached 3.96 (95% CI, 3.63 to 4.32) compared with those with no diabetes. This result was similar to that of a large-scale epidemiological study performed in the United States, which reported that nephritis and nephrotic diseases were the causes of death, and the risk was most increased by the presence of diabetes [8]. Strikingly, proper annual evaluation of renal function (eGFR and albumin-to-creatinine ratio) in patients with type 2 diabetes in primary care clinics was estimated to be only 51.6% in the United States [28]. Moreover, the annual albumin-to-creatinine ratio evaluation rate in Korea was only 28.4% [29]. Therefore, efforts should be made to encourage proper screening and management of renal diseases in primary care clinics.
The large number of participants is a strength of the present study. Taking advantage of the Korean NHIS database, we enrolled 1,292,264 elderly individuals with a median follow-up period of 8.41 years to evaluate mortality characteristics in this study. To the best of our knowledge, the number of participants in our study was the largest compared with that in other studies evaluating mortality characteristics in the elderly population, especially with respect to diabetes [30-32]. Moreover, since the Korean NHIS is a single health insurance cooperation program that mandates nearly all South Koreans to be engaged, we speculate that our study properly represents the general characteristics of the elderly South Korean population. However, this study has some limitations. Hemoglobin A1c and postprandial glucose levels were not included in our study because these parameters are not measured by the National Health Surveillance Program in Korea. Therefore, pre-diabetes patients with impaired glucose tolerance were excluded from this study. However, even without these important laboratory values, the elderly population can be sufficiently classified using administrative information (diagnostic and prescription codes) to stratify the risk of mortality. Hence, we do not believe that these missing laboratory values undermine the results of this study. Recently, novel antidiabetic drugs (sodium-glucose cotransporter-2 inhibitors, glucagon like peptide-1 receptor agonists, and glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide co-agonists) and mineralocorticoid receptor antagonists with proven renal, cardiovascular, or HF benefits in patients with diabetes have been introduced [33-40]. The current study was based on databases for which these drugs were not available. Whether this advancement in medical care can change the characteristics of mortality trends in the elderly population is an interesting subject for future exploration.
In conclusion, we described the characteristics and risk of mortality in an elderly population with respect to the glucose spectrum. We suggest that classifying the elderly population based on their glucose spectrum sufficiently stratifies the risk of mortality. These data provide valuable evidence to support health administration decisions to reduce future healthcare expenses.
Supplementary Material
Supplemental Table S1.
Hazard Ratios and 95% Confidence Intervals for Death according to the Glucose Spectrum (Excluding Cancer Patients)
Supplemental Fig. S1.
Proportions of cause of deaths in elderly people within each category of the glucose spectrum. (A) All-cause mortality. (B) Circulatory system. (C) Respiratory system. (D) Neoplasms. (E) Genitourinary system. (F) Others. I, circulatory system; J, respiratory system; N, genitourinary system; C, neoplasm; O, others; NGT, normal glucose tolerance; IFG, impaired fasting glucose; DM, diabetes mellitus; HR, hazard ratio; CI, confidence interval.
Supplemental Fig. S2.
Risk of all-cause-specific mortality in elderly people according to across the glucose spectrum depending on their underlying diseases in sex subgroups. (A) All-cause mortality. (B) Circulatory system. (C) Respiratory system. (D) Neoplasms. (E) Genitourinary system. (F) Others. CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; IFG, impaired fasting glucose; NGT, normal glucose tolerance.
ACKNOWLEDGMENTS
This study was supported by the Korean Endocrine Society. This study was performed using the database from the National Health Insurance System, and the results do not necessarily represent the opinion of the National Health Insurance Corporation.
REFERENCES
1. Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and global ageing among 65-99-year-old adults: findings from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020; 162:108078.

2. Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J. 2022; 46:417–26.

3. Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th edition Scientific Committee. IDF Diabetes Atlas 2021. 10th ed. Brussels: International Diabetes Federation;2021.
4. Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM; South Tees Diabetes Mortality Study. Cause-specific mortality in a population with diabetes: South Tees Diabetes Mortality Study. Diabetes Care. 2002; 25:43–8.
5. Forbes A, Murrells T, Sinclair AJ. Examining factors associated with excess mortality in older people (age ≥ 70 years) with diabetes: a 10-year cohort study of older people with and without diabetes. Diabet Med. 2017; 34:387–95.
6. Satman I, Bayirlioglu S, Okumus F, Erturk N, Yemenici M, Cinemre S, et al. Estimates and forecasts on the burden of prediabetes and diabetes in adult and elderly population in Turkiye. Eur J Epidemiol. 2023; 38:313–23.

7. Pearson-Stuttard J, Bennett J, Cheng YJ, Vamos EP, Cross AJ, Ezzati M, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021; 9:165–73.

8. Gregg EW, Cheng YJ, Saydah S, Cowie C, Garfield S, Geiss L, et al. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care. 2012; 35:1252–7.
9. Han E, Song SO, Kim HS, Son KJ, Jee SH, Cha BS, et al. Improvement in age at mortality and changes in causes of death in the population with diabetes: an analysis of data from the Korean National Health Insurance and Statistical Information Service, 2006 to 2018. Endocrinol Metab (Seoul). 2022; 37:466–74.

10. Cho SW, Kim JH, Choi HS, Ahn HY, Kim MK, Rhee EJ. Big data research in the field of endocrine diseases using the Korean National Health Information Database. Endocrinol Metab (Seoul). 2023; 38:10–24.

11. Kim MK, Han K, Lee SH. Current trends of big data research using the Korean National Health Information Database. Diabetes Metab J. 2022; 46:552–63.

12. Pilotto A, Ferrucci L, Franceschi M, D’Ambrosio LP, Scarcelli C, Cascavilla L, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008; 11:151–61.

13. Baek JH, Park YM, Han KD, Moon MK, Choi JH, Ko SH. Comparison of operational definition of type 2 diabetes mellitus based on data from Korean National Health Insurance Service and Korea National Health and Nutrition Examination Survey. Diabetes Metab J. 2023; 47:201–10.

14. Lee SH, Yu J, Han K, Lee SW, You SY, Kim HS, et al. Predicting the risk of insulin-requiring gestational diabetes before pregnancy: a model generated from a nationwide population-based cohort study in Korea. Endocrinol Metab (Seoul). 2023; 38:129–38.

15. Kang YM, Kim YJ, Park JY, Lee WJ, Jung CH. Mortality and causes of death in a national sample of type 2 diabetic patients in Korea from 2002 to 2013. Cardiovasc Diabetol. 2016; 15:131.

16. Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GC, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020; 22:1607–18.

17. Chen R, Ovbiagele B, Feng W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci. 2016; 351:380–6.

18. Rose S. Mortality risk score prediction in an elderly population using machine learning. Am J Epidemiol. 2013; 177:443–52.

19. Hong Kong Diabetes Registry, Yang X, So WY, Tong PC, Ma RC, Kong AP, et al. Development and validation of an all-cause mortality risk score in type 2 diabetes. Arch Intern Med. 2008; 168:451–7.

20. Ravaglia G, Forti P, Lucicesare A, Pisacane N, Rietti E, Patterson C. Development of an easy prognostic score for frailty outcomes in the aged. Age Ageing. 2008; 37:161–6.

21. Nierman DM, Schechter CB, Cannon LM, Meier DE. Outcome prediction model for very elderly critically ill patients. Crit Care Med. 2001; 29:1853–9.

22. Choi G, Yoon H, Choi HH, Ha KH, Kim DJ. Association of prediabetes with death and diabetic complications in older adults: the pros and cons of active screening for prediabetes. Age Ageing. 2022; 51:afac116.

23. Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-specific trends from 2000-2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care. 2016; 39:1018–26.

24. Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004; 109:1101–7.

25. Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007; 356:2388–98.

26. Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005; 2:48–53.

27. Scully T, Ettela A, LeRoith D, Gallagher EJ. Obesity, type 2 diabetes, and cancer risk. Front Oncol. 2021; 10:615375.

28. Stempniewicz N, Vassalotti JA, Cuddeback JK, Ciemins E, Storfer-Isser A, Sang Y, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. health care organizations. Diabetes Care. 2021; 44:2000–9.

29. Seo DH, Kang S, Lee YH, Ha JY, Park JS, Lee BW, et al. Current management of type 2 diabetes mellitus in primary care clinics in Korea. Endocrinol Metab (Seoul). 2019; 34:282–90.

30. Chang YK, Huang LF, Shin SJ, Lin KD, Chong K, Yen FS, et al. A point-based mortality prediction system for older adults with diabetes. Sci Rep. 2017; 7:12652.

31. Muggeo M, Verlato G, Bonora E, Ciani F, Moghetti P, Eastman R, et al. Long-term instability of fasting plasma glucose predicts mortality in elderly NIDDM patients: the Verona Diabetes Study. Diabetologia. 1995; 38:672–9.

32. Hiltunen L, Laara E, Kivela SL, Keinanen-Kiukaanniemi S. Glucose tolerance and mortality in an elderly Finnish population. Diabetes Res Clin Pract. 1998; 39:75–81.

33. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020; 383:2219–29.

34. Heerspink HJ, Sattar N, Pavo I, Haupt A, Duffin KL, Yang Z, et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: posthoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022; 10:774–85.

35. The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023; 388:117–27.

36. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJ, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–306.

37. Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–46.

38. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019; 394:131–8.
Fig. 1.
Risk of cause-specific mortality in elderly people according to the glucose spectrum in age subgroups. (A) All-cause mortality. (B) Circulatory system. (C) Respiratory system. (D) Neoplasms. (E) Genitourinary system. (F) Others. NGT, normal glucose tolerance; IFG, impaired fasting glucose; DM, diabetes mellitus; HR, hazard ratio; CI, confidence interval.
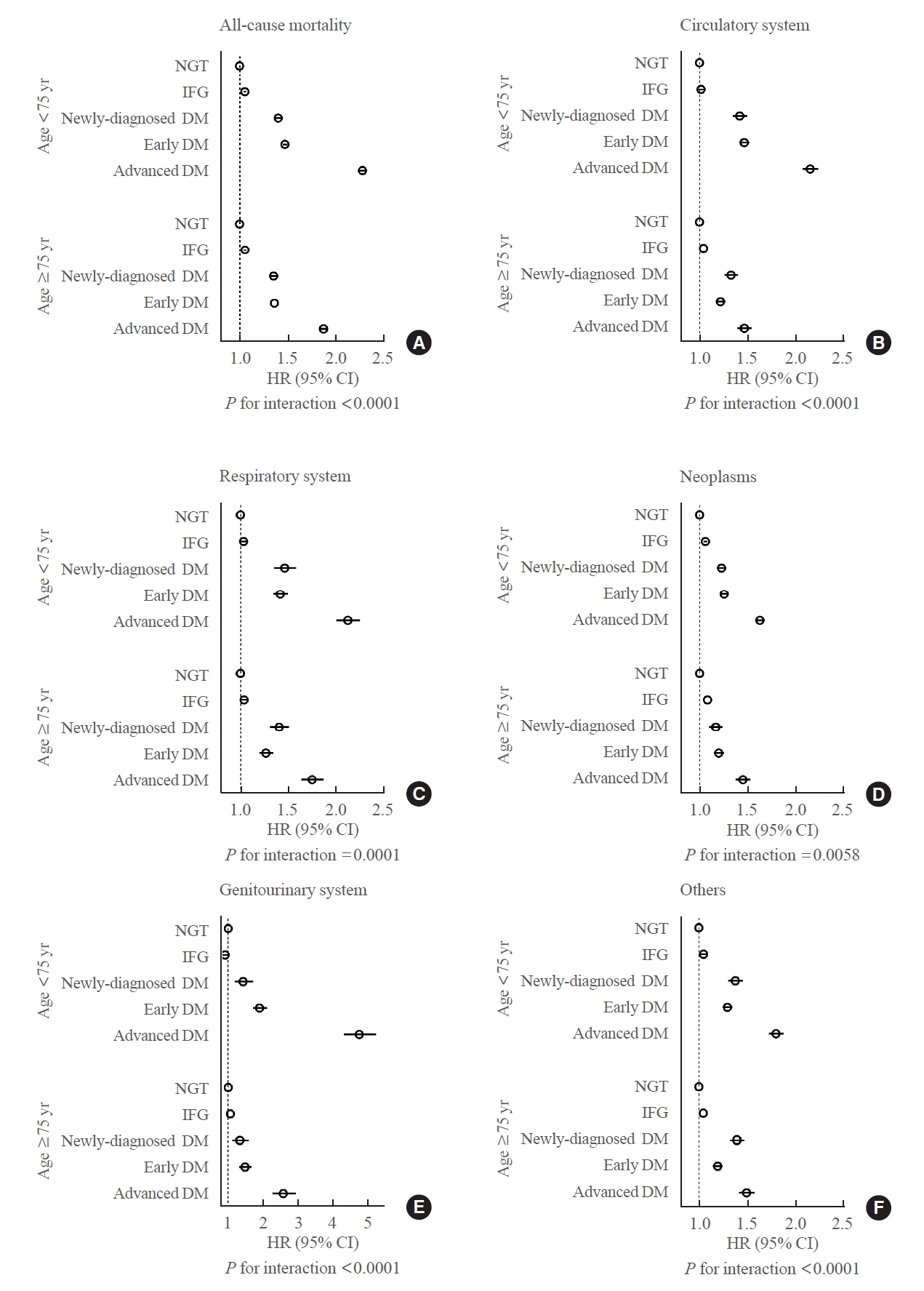
Fig. 2.
Risk of all-cause mortality in elderly people across the glucose spectrum depending on their underlying diseases: (A) stroke, (B) heart failure (HF), and (C) chronic kidney disease (CKD). HR, hazard ratio; CI, confidence interval; NGT, normal glucose tolerance; IFG, impaired fasting glucose; DM, diabetes mellitus.
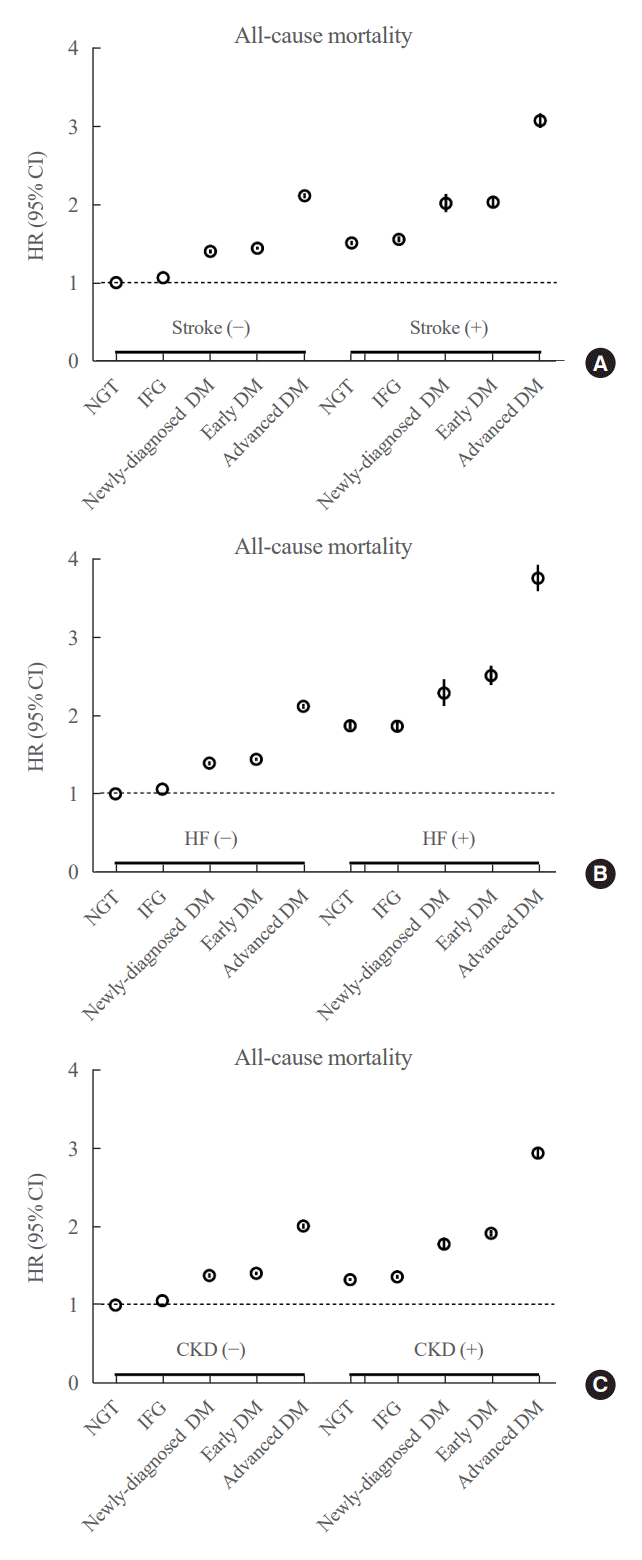
Table 1.
Baseline Characteristics of the Study Population across the Glucose Spectrum
Values are expressed as mean±standard deviation, number (%) or median (interquartile range). P values for the trend were <0.0001 for all variables.
NGT, normal glucose tolerance; IFG, impaired fasting glucose; DM, diabetes mellitus; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 2.
Hazard Ratios and 95% Confidence Intervals for Death according to the Glucose Spectrum
Table 3.
Hazard Ratios and 95% Confidence Intervals for Specific Cause of Death according to the Glucose Spectrum




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download