2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017; 390:2627–42.
3. Ha KH, Kim DJ. Epidemiology of childhood obesity in Korea. Endocrinol Metab (Seoul). 2016; 31:510–8.

4. Rhie S, Lee S, Chae KY. Sleep patterns and school performance of Korean adolescents assessed using a Korean version of the pediatric daytime sleepiness scale. Korean J Pediatr. 2011; 54:29–35.

5. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010; 33:585–92.

6. Kim BK, Kim BS, An SY, Lee MS, Choi YJ, Han SJ, et al. Sleep duration and glycemic control in patients with diabetes mellitus: Korea National Health and Nutrition Examination Survey 2007-2010. J Korean Med Sci. 2013; 28:1334–9.

7. Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008; 1129:287–304.

8. Kohansieh M, Makaryus AN. Sleep deficiency and deprivation leading to cardiovascular disease. Int J Hypertens. 2015; 2015:615681.

9. Sato K, Meng F, Francis H, Wu N, Chen L, Kennedy L, et al. Melatonin and circadian rhythms in liver diseases: functional roles and potential therapies. J Pineal Res. 2020; 68:e12639.

10. Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006; 29:657–61.

11. Yeo Y, Ma SH, Park SK, Chang SH, Shin HR, Kang D, et al. A prospective cohort study on the relationship of sleep duration with all-cause and disease-specific mortality in the Korean Multi-center Cancer Cohort study. J Prev Med Public Health. 2013; 46:271–81.

12. Rodriguez V, Mellado C, Alvarez E, De Diego JG, Blazquez E. Effect of pinealectomy on liver insulin and glucagon receptor concentrations in the rat. J Pineal Res. 1989; 6:77–88.

13. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014; 56:371–81.

14. Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 2013; 98:337–44.

15. Szewczyk-Golec K, Wozniak A, Reiter RJ. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: implications for obesity. J Pineal Res. 2015; 59:277–91.

16. She M, Laudon M, Yin W. Melatonin receptors in diabetes: a potential new therapeutical target? Eur J Pharmacol. 2014; 744:220–3.

17. Xu P, Wang J, Hong F, Wang S, Jin X, Xue T, et al. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017; 62:e12399.

18. Duan Y, Gong K, Xu S, Zhang F, Meng X, Han J. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal Transduct Target Ther. 2022; 7:265.

19. Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol. 2011; 73:239–59.

20. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020; 21:225–45.

21. Wang L, McFadden JW, Yang G, Zhu H, Lian H, Fu T, et al. Effect of melatonin on visceral fat deposition, lipid metabolism and hepatic lipo-metabolic gene expression in male rats. J Anim Physiol Anim Nutr (Berl). 2021; 105:787–96.

22. Mi Y, Tan D, He Y, Zhou X, Zhou Q, Ji S. Melatonin modulates lipid metabolism in HepG2 cells cultured in high concentrations of oleic acid: AMPK pathway activation may play an important role. Cell Biochem Biophys. 2018; 76:463–70.

23. Hussain SA. Effect of melatonin on cholesterol absorption in rats. J Pineal Res. 2007; 42:267–71.

24. Michurina SV, Kolesnikov SI, Ishchenko IY, Arkhipov SA. Light-induced functional pinealectomy: expression of MT2 receptors in liver cells of C57BL/6 mice after melatonin treatment. Bull Exp Biol Med. 2022; 173:569–74.

25. Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol. 2011; 17:3888–98.

26. Tung YT, Chiang PC, Chen YL, Chien YW. Effects of melatonin on lipid metabolism and circulating irisin in Sprague-Dawley rats with diet-induced obesity. Molecules. 2020; 25:3329.

27. Keskin I, Kaplan S, Kalkan S, Sutcu M, Ulkay MB, Esener OB. Evaluation of neuroprotection by melatonin against adverse effects of prenatal exposure to a nonsteroidal anti-inflammatory drug during peripheral nerve development. Int J Dev Neurosci. 2015; 41:1–7.

28. Onger ME, Kaplan S, Deniz OG, Altun G, Altunkaynak BZ, Balci K, et al. Possible promoting effects of melatonin, leptin and alcar on regeneration of the sciatic nerve. J Chem Neuroanat. 2017; 81:34–41.

29. Mirza MS. Obesity, visceral fat, and NAFLD: querying the role of adipokines in the progression of nonalcoholic fatty liver disease. ISRN Gastroenterol. 2011; 2011:592404.
30. Sun H, Huang FF, Qu S. Melatonin: a potential intervention for hepatic steatosis. Lipids Health Dis. 2015; 14:75.

31. Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, Renard P, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology. 2003; 144:5347–52.

32. Shieh JM, Wu HT, Cheng KC, Cheng JT. Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKCzeta-Akt-GSK3beta pathway in hepatic cells. J Pineal Res. 2009; 47:339–44.
33. Behbodikhah J, Ahmed S, Elyasi A, Kasselman LJ, De Leon J, Glass AD, et al. Apolipoprotein B and cardiovascular disease: biomarker and potential therapeutic target. Metabolites. 2021; 11:690.

34. Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009; 296:E1183–94.

35. Mohammadi-Sartang M, Ghorbani M, Mazloom Z. Effects of melatonin supplementation on blood lipid concentrations: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018; 37(6 Pt A):1943–54.

36. Mathes AM. Hepatoprotective actions of melatonin: possible mediation by melatonin receptors. World J Gastroenterol. 2010; 16:6087–97.

37. Gomez-Lechon MJ, Donato MT, Martinez-Romero A, Jimenez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007; 165:106–16.

38. Park JY, Kim Y, Im JA, Lee H. Oligonol suppresses lipid accumulation and improves insulin resistance in a palmitateinduced in HepG2 hepatocytes as a cellular steatosis model. BMC Complement Altern Med. 2015; 15:185.

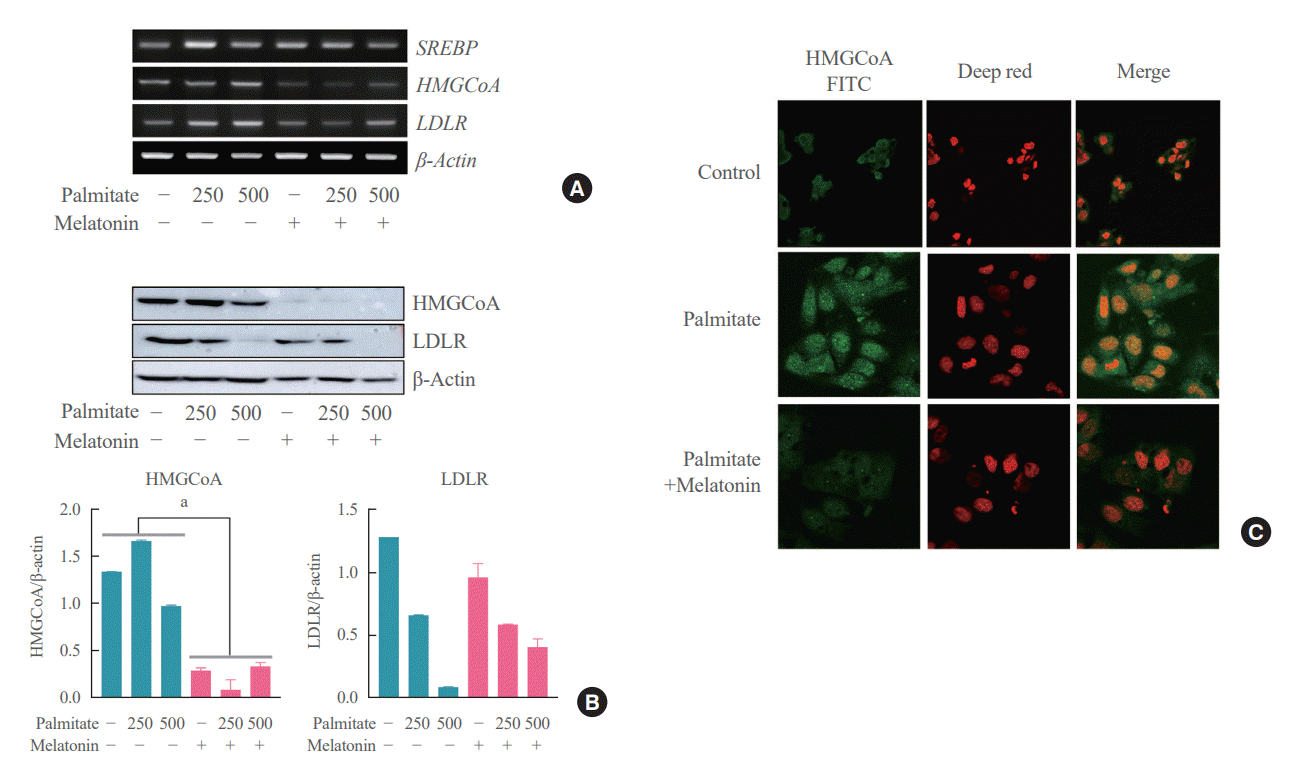




 PDF
PDF Citation
Citation Print
Print



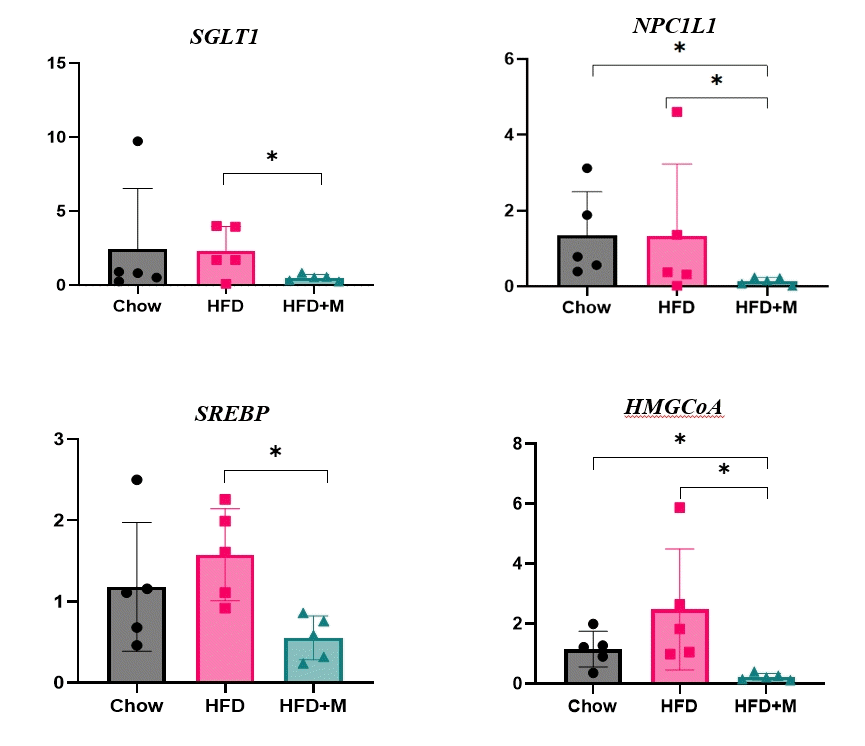
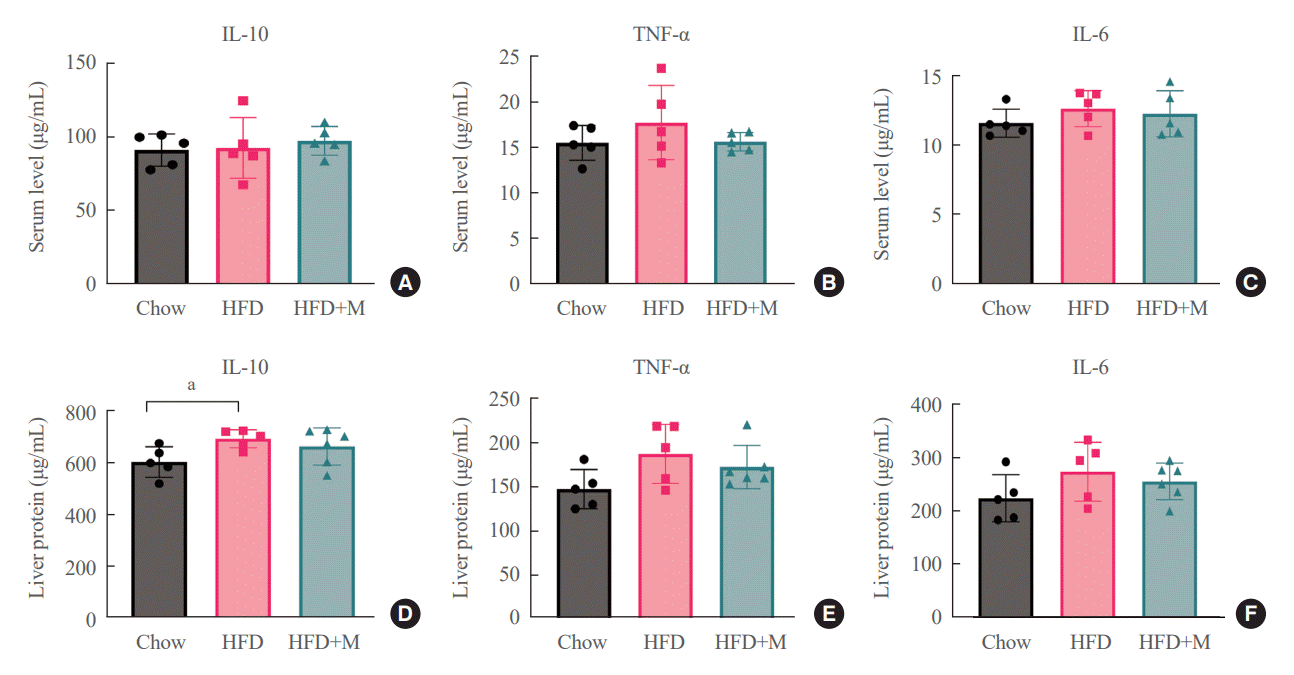
 XML Download
XML Download