Abstract
Background
Thyroid cancer mortality has been largely overlooked as relatively stable given the large gap between thyroid cancer incidence and mortality. This study evaluated long-term trends in age-standardized mortality rates (ASMRs) throughout Korea and compared them with mortality data reported by the Surveillance, Epidemiology, and End Results (SEER).
Methods
Cancer-specific mortality data from 1985 to 2020 were obtained from Statistics Korea. ASMRs from thyroid cancer were calculated based on the Korean mid-year resident registration population of 2005. We assessed SEER*Explorer and downloaded the mortality data.
Results
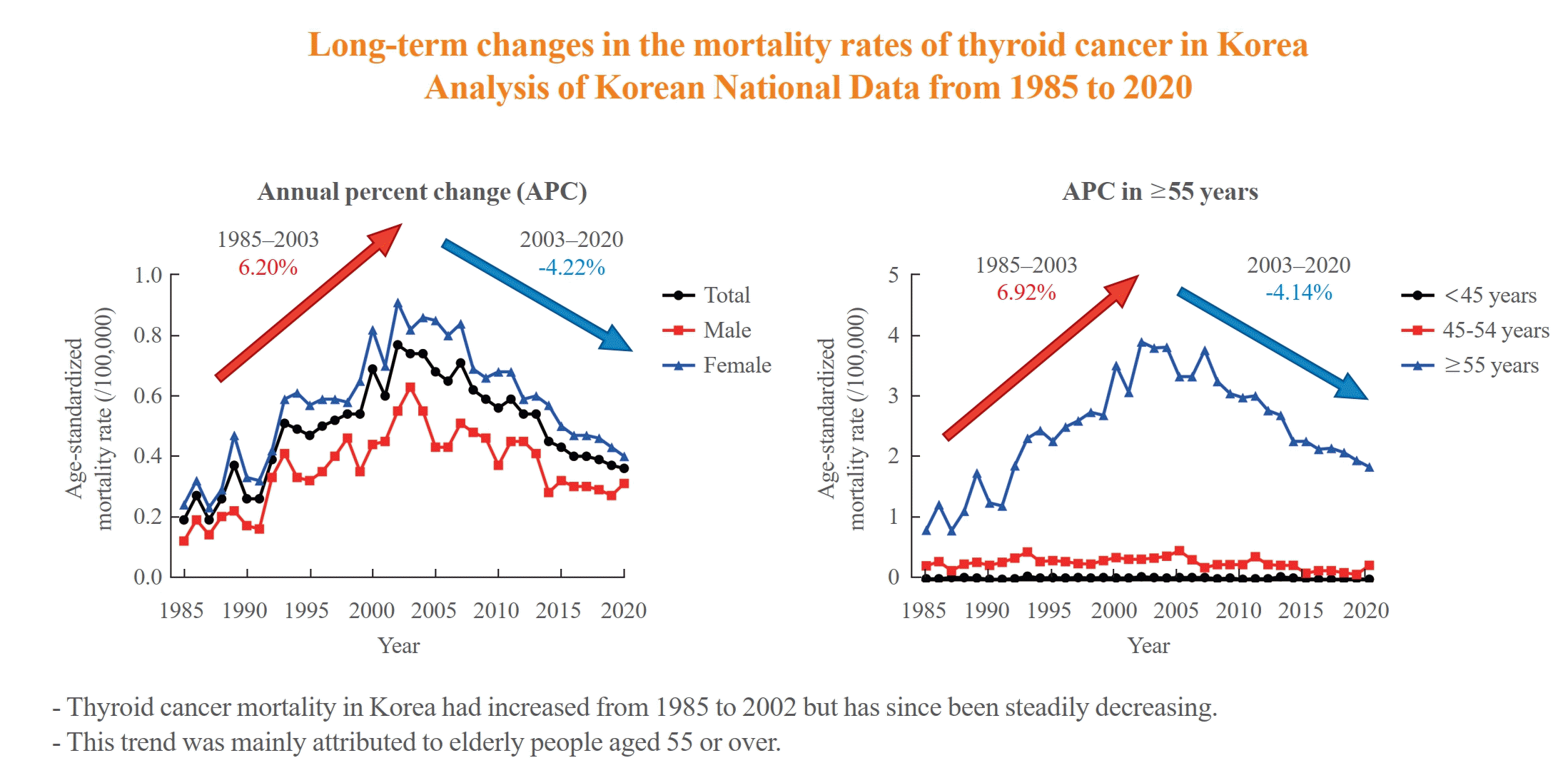
The ASMR increased from 0.19 to 0.77/100,000 between 1985 and 2002 but decreased continuously to 0.36/100,000 in 2020. The annual percent change (APC) in the ASMR between 1985 and 2003 and between 2003 and 2020 was 6.204 and −4.218, respectively, with similar patterns observed in both men and women. The ASMR of the SEER showed a modest increase from 1988 to 2016 and then stabilized. In subgroup analysis, the ASMR of the old age group (≥55 years) increased significantly from 0.82 in 1985 to 3.92/100,000 in 2002 (APC 6.917) but then decreased again to 1.86/100,000 in 2020 (APC −4.136). ASMRs according to the age group in the SEER showed a relatively stable trend even in the elderly group.
Thyroid cancer is the most common endocrine malignancy and the fastest-increasing cancer worldwide [1,2]. Research has recognized that the substantial increase in the incidence of thyroid cancer could be attributed to increased detection and overdiagnosis. However, true increases in incidence rates due to enhanced exposure to selected risk factors cannot be overlooked [3,4]. Although South Korea has had the most prominent increase in thyroid cancer incidence rate, recent data have shown a decline after 2012 followed by stabilization [5]. Monitoring trends in population-based data on cancer burden is critical and provides insights into epidemiological patterns that might be influenced by both environmental and systemic effects [6,7]. Nonetheless, we believe mortality is the most important parameter among several measures that have been developed to address cancer control and quality of care [7]. Changing incidence must be interpreted in conjunction with mortality and knowledge about changing diagnostic practice [7].
Several studies have investigated the changes in thyroid cancer mortality rates, with their findings suggesting varying trends according to geographic region and period [8-12]. Changes in mortality have been considered as relatively stable given the large gap between thyroid cancer incidence and mortality [8]. However, a recent study using incidence-based mortality data by the Surveillance, Epidemiology, and End Results (SEER) program showed that mortality is increasing [10]. On the other hand, another study using age–period–cohort analysis showed decreasing international thyroid cancer mortality [11].
Considering that South Korea has experienced the most dramatic changes in the incidence of thyroid cancer, evaluating trends in the mortality rate of thyroid cancer throughout Korea will be of great significance. Previously, we reported long-term trends of thyroid cancer mortality rates in Korea from 1985 to 2015 [13]. The epidemiological situation of thyroid cancer has changed significantly due to various external factors since the 2010s. Therefore, it is necessary to further investigate the change in thyroid cancer mortality, and it will be important to compare and analyze it with data from the other country.
In this study, we extended the period until 2020 to look at mortality trends until recently. Additionally, we compared trends in Korean mortality from thyroid cancer with the mortality data from the SEER program.
Cancer-specific mortality data from 1985 to 2020 were obtained from Statistics Korea [14]. Data were downloaded on January 1, 2022. The cause of death was coded and classified according to the International Classification for Diseases, 10th edition code for thyroid cancer (C73). We assessed SEER*Explorer and downloaded the omortality data on October 9, 2022 [15]. The study protocol was approved by the Institutional Review Board (IRB) of Hallym University Dongtan Sacred Heart Hospital, Korea (IRB no. HDT 2022-04-015).
The crude mortality rates (CMRs) and age-standardized mortality rates (ASMRs) for thyroid cancer were calculated. ASMRs were determined using the Korean mid-year resident registration population of 2005 (defined as the standard Korean population). Subanalysis of the mortality rates was conducted according to sex and age, with the following age categories being used: <45, 45–54, or ≥55 years.
We performed joinpoint regression analysis allowing for a maximum of two joinpoints using a log-linear model to identify significant changes in mortality trends [16]. For each period of the identified trends, we calculated the estimated annual percent change (APC). Because the SEER program data is standardized with a different standard population, we compared each trend without comparing the absolute values. P values of less than 0.05 indicated statistical significance. Statistical analyses were performed using the Joinpoint Regression Program version 4.8.0.1 (Statistical Research and Application Branch, National Cancer Institute, Bethesda, MD, USA).
The CMR of thyroid cancer was 0.11 (95% confidence interval [CI], 0.08 to 0.15) per 100,000 in 1985, which increased to 0.78 (95% CI, 0.70 to 0.86) per 100,000 in 2007 (Supplemental Table S1, Supplemental Fig. S1A) and then stabilized thereafter. This trend was similar in both men and women (Supplemental Table S1, Supplemental Fig. S1A). In the older subgroup (aged ≥55 years), the CMR was 0.80 (95% CI, 0.55 to 1.13) per 100,000 in 1985 peaked at 3.90 (95% CI, 3.51 to 4.33) per 100,000 in 2007, after which it decreased to 2.12 by 2020 (95% CI, 1.91 to 2.36) (Supplemental Table S1, Supplemental Fig. S1B).
The ASMR increased from 0.19 (95% CI, 0.15 to 0.23) to 0.77 (95% CI, 0.69 to 0.85) per 100,000 between 1985 and 2002. However, it decreased continuously to 0.36 (95% CI, 0.31 to 0.42) per 100,000 by 2020 (Table 1, Fig. 1A). The APC of the ASMR was 6.204 (95% CI, 5.034 to 7.387; P<0.01) between 1985 and 2003 and −4.218 (95% CI, −5.047 to −3.381; P<0.01) that between 2003 and 2020 (Table 2, Fig. 1B). The average annual percent change (AAPC) from 1985 to 2020 was 1.008 (95% CI, 0.322 to 1.698; P=0.004). These trends in APC exhibited similar patterns in both men and women. In males, the ASMR increased from 0.12 (95% CI, 0.09 to 0.16) to 0.63 (95% CI, 0.56 to 0.70) per 100,000 between 1985 and 2003 (APC, 6.198 [95% CI, 4.401 to 8.026; P<0.01]) and then decreased to 0.31 (95% CI, 0.26 to 0.36) per 100,000 in 2020 (APC, −4.084 [95% CI, −5.299 to −2.853; P<0.01]; AAPC, 1.073 [95% CI, 0.035 to 2.122; P<0.01]) (Fig. 1C). In female, the ASMR increased from 0.24 (95% CI, 0.20 to 0.28) to 0.91 (95% CI, 0.83 to 1.00) per 100,000 between 1985 and 2004 (APC, 5.439 [95% CI, 4.309 to 6.582; P<0.01]) and then decreased to 0.40 (95% CI, 0.34 to 0.46) per 100,000 in 2020 (APC, −4.910 [95% CI, −5.936 to −3.872; P<0.01]; AAPC, 0.576 [95% CI, −0.163 to 1.320; P=0.13]) (Fig. 1D). However, the AAPC was only significantly increased in men (1.073 [95% CI, 0.035 to 2.122; P<0.01]) and not women (0.576 [95% CI, −0.163 to 1.320; P=0.127]) (Table 2, Fig. 1C, D). Conversely, the ASMR trend of the SEER program was modest despite its significant APC. The ASMR increased from 0.44 (95% CI, 0.41 to 0.47) to 0.54 (95% CI, 0.51 to 0.56) per 100,000 between 1988 and 2016 but then decreased to 0.48 (95% CI, 0.46 to 0.50) per 100,000 by 2020 (Fig. 1E). The APC of the ASMR was 0.7 (95% CI, 0.5 to 0.9; P<0.01) between 1988 and 2016 and −1.9 (95% CI, −4.3 to 0.5; P=0.12) between 2016 and 2020 (Fig. 1E). These trends in APC exhibited similar patterns in both men and women.
We evaluated the ASMR according three age groups (Table 1, Fig. 2A). The ASMR of youngest age group (under 45 years) was very low and remained stable (APC, −0.403 [95% CI, −0.159 to 0.799; P=0.498, between 1985 and 2020]) (Table 2, Fig. 2B). Moreover, the ASMR of those between 45 and 54 years showed modest yet significant changes. The APC was 1.630 (95% CI, 0.116 to 3.167; P=0.036) between 1985 and 2005 and −5.146 (95% CI, −8.782 to −1.364; P=0.010) between 2005 and 2020 (Table 2, Fig. 2C). The AAPC throughout the entire period was not significant (−1.331 [95% CI, −3.100 to 0.470; P=0.146). Of note, the ASMR of the old age group (from 55 years) changed significantly from 0.82 (95% CI, 0.64 to 1.04) in 1985 to 3.92 (95% CI, 3.51 to 4.37) per 100,000 in 2002 and subsequently decreased to 1.86 (95% CI, 1.57 to 2.17) per 100,000 by 2020. The APC was 6.917 (95% CI, 5.647 to 8.203; P<0.001) between 1985 and 2003 and −4.136 (95% CI, −4.936 to −3.329; P<0.001) between 2003 and 2020 (Table 2, Fig. 2D). The AAPC increased significantly throughout the entire period (1.398 [95% CI, 0.682 to 2.119; P<0.001]). Likewise, the ASMRs of the young age groups (under 65 years) included in the SEER program were very low (Fig. 2E), with that of the elderly group (≥65 years) also showing a relatively stable trend. The APC of the elderly group was 1.1 (95% CI, 0.7 to 1.4; P<0.01) between 1989 and 2009 and −0.1 (95% CI, −0.7 to 0.6; P=0.84) between 2009 and 2020.
This study investigated the long-term trends in the ASMR of thyroid cancer throughout Korea. Accordingly, our findings showed that the overall ASMR increased from 0.19 per 100,000 in 1985 to 0.77 per 100,000 in 2002 and began to decline to 0.36 per 100,000 by 2020, with similar trends having been observed for both sexes. Of note, this trend in the ASMR of thyroid cancer was mainly derived from elderly people aged 55 or over. Among those aged 55 years old or older, the ASMR increased from 0.82 per 100,000 in 1985 to 3.92 per 100,000 in 2002 and then decreased to 1.86 per 100,000 in 2020. In contrast, the overall ASMR of United States SEER data showed a modest change of around 0.5 per 100,000. The peak of the ASMR was higher for Korean data than for SEER data. During subgroup analysis according to age groups, SEER data also showed that most of the deaths occurred in elderly people aged 65 or over, with modest changes compared to Korean data (between 2.5 and 3.5 per 100,000) throughout the studied period. In addition, the mortality rate is higher in females than in males over the entire period in Korean data. Since the number of deaths is small and the difference is not consistent across the years, it seems difficult to explain this phenomenon. However, it seems that overall mortality trends tend to be affected more by females than males.
Analysis of thyroid cancer mortality rates have yielded inconsistent results according to geographic area, periods included, and analytic methods [17-19]. The current analysis on Korean mortality were based on death certificates, which did not contain incidence-based data, such as age at diagnosis and stage of disease. Nonetheless, Lim et al. [10] who evaluated the incidence-based mortality of SEER-9, found an overall increase of 1.1% annually. Thereafter, several papers had also reported on the incidence-based mortality of the SEER program, subsequently showing a similar increasing trend [12,20-22]. However, another study by Li et al. [11] who conducted age–period–cohort analysis for international thyroid cancer mortality, found that long-term rates declined over time in most countries, converging around a value of 0.5/100,000. Overall, inconsistencies still surround the estimation and interpretation of the changes in thyroid cancer mortality.
In Korea, the age-standardized incidence of thyroid cancer had steadily increased from 1999, during which the Korea National Cancer Incidence Database started to report nationwide statistics, up to 2012 [5]. During the 1990s, thyroid ultrasonography and fine-needle aspiration had begun to spread, with Korean government initiating a national cancer screening program from 1999. Some investigators have suggested that a substantial proportion of this increase could have been attributed to overdiagnosis; however, the ASMR of thyroid cancer had continued to increase since 1985 before the launch of the national cancer screening program. This ASMR, which had been steadily increasing for 20 years, began to decrease around 2005. This could probably be attributed to the discovery of advanced-stage cancers as well as small cancers and the development of thyroid cancer treatment. This was followed by a decrease in incidence rate from 2012 before the debates regarding overdiagnosis of thyroid cancer began in 2014 in Korea [5,23].
Of note, the peak of the ASMR was higher for Korean data than for SEER data. Furthermore, the absolute value of the APC for Korean data was much higher than many other previous thyroid cancer mortality studies. Lim et al. [10] reported a 1.1% annual increase in incidence-based mortality from 1994 to 2013 based on SEER-9. More recently, Megwalu and Moon [12] reported a 1.35% annual increase in incidence-based mortality from 2000 to 2018 based on SEER-18. In contrast, the overall APC in Korea was 6.20 between 1985 and 2003 and −4.22 between 2003 and 2020. The reason for this high mortality rate and large APC is currently difficult to determine. In general, however, mortality has been known to have little effect on bias caused by screening, suggesting that there must be an intrinsic factor explaining such a large change. Several studies have reported an increase in point mutations, such as BRAF or RAS, which are more likely to have a nonradiation etiology, accompanied by increasing thyroid cancer incidence [4,24,25]. Although BRAF mutations have been widely accepted to be correlated with moderately worse prognosis, the prognostic value of BRAF mutations in isolation remains inconclusive [26]. Accordingly, they found that the prevalence of BRAF mutations was significantly higher in Asia, especially in Korea (ranging from 60% to 87%) [27-29]. Future consideration is needed to determine whether these genetic changes are associated with changes in mortality and incidence.
In a study by Dong et al. [30], the annual hazard curve for thyroid cancer mortality presented a bimodal distribution, with the first peak at the 10th year and the second at the 20th year after surgery. In other words, there are persistent risk of cancer mortality even 10 or more years after initial treatment. Due to concerns regarding overdiagnosis and the resulting changes in guidelines for the diagnosis and treatment of thyroid cancer, the incidence of thyroid cancer in Korea has been declining since 2012. Continuous long-term follow-up is needed to determine the effects of these changes on mortality rates in the future.
This study has several limitations. First, our mortality data were based only on death certificates, which lacked disease-specific information such as age at diagnosis and disease stage. Furthermore, we lacked data on tumor histology. Second, owing to the descriptive nature of this study, we could only speculate on the potential explanations for the observed thyroid cancer trends. Third, we used different standard population from the SEER program. Thus, we can only compare each trend rather than absolute mortality rates. Fourth, there is also a difference in the age criterion for the subanalysis between our data and the SEER program. We classified age as <55 or ≥55 years according to an eighth edition of the Union for International Cancer Control/American Joint Committee on Cancer, tumor, node and metastasis (TNM) staging system for thyroid cancer. However, in the SEER program, a subanalysis was performed by the forementioned age group across all types of carcinomas. We reported the results as they are according to the criteria presented in the SEER program data.
In conclusion, the ASMR of thyroid cancer in Korea had increased from 1985 to 2002, after which it has continued to decrease. This trend was mainly derived from elderly people aged 55 or over. Compared to SEER data, the absolute APC value for Korean data was much higher. However, determining the reasons for these changes in mortality trends and differences among countries has still been difficult. Given that the time lag from any changes in certain environmental factors or healthcare systems to reflect in epidemiologic indicators is greater in thyroid cancer than in any other cancer type, future continuous and longer follow-up mortality studies that integrate incidence data are needed.
Supplementary Material
Supplemental Table S1.
Crude Mortality Rates (per 100,000) of Thyroid Cancer from 1985 to 2020 in Korea
Supplemental Fig. S1.
Crude mortality rates of thyroid cancer in Korea. (A) Overall and according to sex, (B) according to age.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: Y.M.C., W.B.K., W.G.K. Acquisition, analysis, or interpretation of data: Y.M.C., M.J.K., J.L., M.K.K., M.J.J. Drafting the work or revising: Y.M.C., T.Y.K., E.G.H., W.B.K., W.G.K. Final approval of the manuscript: Y.M.C., M.J.K., J.L., M.K.K., M.J.J., T.Y.K., E.G.H., W.B.K., W.G.K.
ACKNOWLEDGMENTS
This work was supported by a grant (2023IL0010) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
REFERENCES
1. Kitahara CM, Schneider AB. Epidemiology of thyroid cancer. Cancer Epidemiol Biomarkers Prev. 2022; 31:1284–97.

2. Seib CD, Sosa JA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am. 2019; 48:23–35.

3. Kitahara CM, Sosa JA. Understanding the ever-changing incidence of thyroid cancer. Nat Rev Endocrinol. 2020; 16:617–8.

4. Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA Jr, Sigurdson AJ, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014; 99:E276–85.
5. Choi YM, Lee J, Kwak MK, Jeon MJ, Kim TY, Hong EG, et al. Recent changes in the incidence of thyroid cancer in Korea between 2005 and 2018: analysis of Korean National Data. Endocrinol Metab (Seoul). 2022; 37:791–9.

6. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022; 10:264–72.

7. Welch HG, Kramer BS, Black WC. Epidemiologic signatures in cancer. N Engl J Med. 2019; 381:1378–86.

8. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014; 140:317–22.

9. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015; 136:2187–95.

10. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017; 317:1338–48.

11. Li M, Brito JP, Vaccarella S. Long-term declines of thyroid cancer mortality: an international age-period-cohort analysis. Thyroid. 2020; 30:838–46.

12. Megwalu UC, Moon PK. Thyroid cancer incidence and mortality trends in the United States: 2000-2018. Thyroid. 2022; 32:560–70.

13. Choi YM, Kim WG, Kwon H, Jeon MJ, Han M, Kim TY, et al. Changes in standardized mortality rates from thyroid cancer in Korea between 1985 and 2015: analysis of Korean national data. Cancer. 2017; 123:4808–14.

14. Korean Statistical Information Service. Causes of death statistics [Internet]. Daejeon: Statistics Korea;2022. [cited 2023 Jul 28]. Available from: https://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01&outLink=Y&entrType=.
15. Surveillance Research Program. Surveillance, Epidemiology, and End Results. SEER*Ex-plorer: an interactive website for SEER cancer statistics [Internet]. Bethesda: National Cancer Institute;2022. [cited 2023 Jul 28]. Available from: https://seer.cancer.gov/statistics-network/explorer/.
16. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000; 19:335–51.

17. Deng Y, Li H, Wang M, Li N, Tian T, Wu Y, et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw Open. 2020; 3:e208759.

18. Wang J, Yu F, Shang Y, Ping Z, Liu L. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine. 2020; 68:163–73.

19. Wirth S, Syleouni ME, Karavasiloglou N, Rinaldi S, Korol D, Wanner M, et al. Incidence and mortality trends of thyroid cancer from 1980 to 2016. Swiss Med Wkly. 2021; 151:w30029.

20. LaBarge B, Walter V, Bann DV, Goldenberg D. In-depth analysis of thyroid cancer mortality. Head Neck. 2021; 43:977–83.

21. Rahimi L, Brito JP. US thyroid cancer incidence fell but mortality climbing in recent years. Clin Thyroidol. 2022; 34:213–5.

22. Yan KL, Li S, Tseng CH, Kim J, Nguyen DT, Dawood NB, et al. Rising incidence and incidence-based mortality of thyroid cancer in California, 2000-2017. J Clin Endocrinol Metab. 2020; 105:dgaa121.

23. Oh CM, Lim J, Jung YS, Kim Y, Jung KW, Hong S, et al. Decreasing trends in thyroid cancer incidence in South Korea: what happened in South Korea? Cancer Med. 2021; 10:4087–96.

24. Romei C, Fugazzola L, Puxeddu E, Frasca F, Viola D, Muzza M, et al. Modifications in the papillary thyroid cancer gene profile over the last 15 years. J Clin Endocrinol Metab. 2012; 97:E1758–65.

25. Kowalska A, Walczyk A, Kowalik A, Palyga I, Trybek T, Kopczynski J, et al. Increase in papillary thyroid cancer incidence is accompanied by changes in the frequency of the BRAF V600E mutation: a single-institution study. Thyroid. 2016; 26:543–51.

26. Haymart MR. Is BRAF V600E mutation the explanation for age-associated mortality risk in patients with papillary thyroid cancer? J Clin Oncol. 2018; 36:433–4.
27. Song YS, Lim JA, Park YJ. Mutation profile of well-differentiated thyroid cancer in Asians. Endocrinol Metab (Seoul). 2015; 30:252–62.

28. Kim KH, Kang DW, Kim SH, Seong IO, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J. 2004; 45:818–21.

Fig. 1.
(A) Age-standardized mortality rates (ASMR) of thyroid cancer in Korea according to sex. Annual percent change (APC) of ASMR in (B) all subjects, (C) males, and (D) females. (E) Long-term trends in United States ASMR of thyroid cancer in the Surveillance, Epidemiology, and End Results program. aStatistically significant.
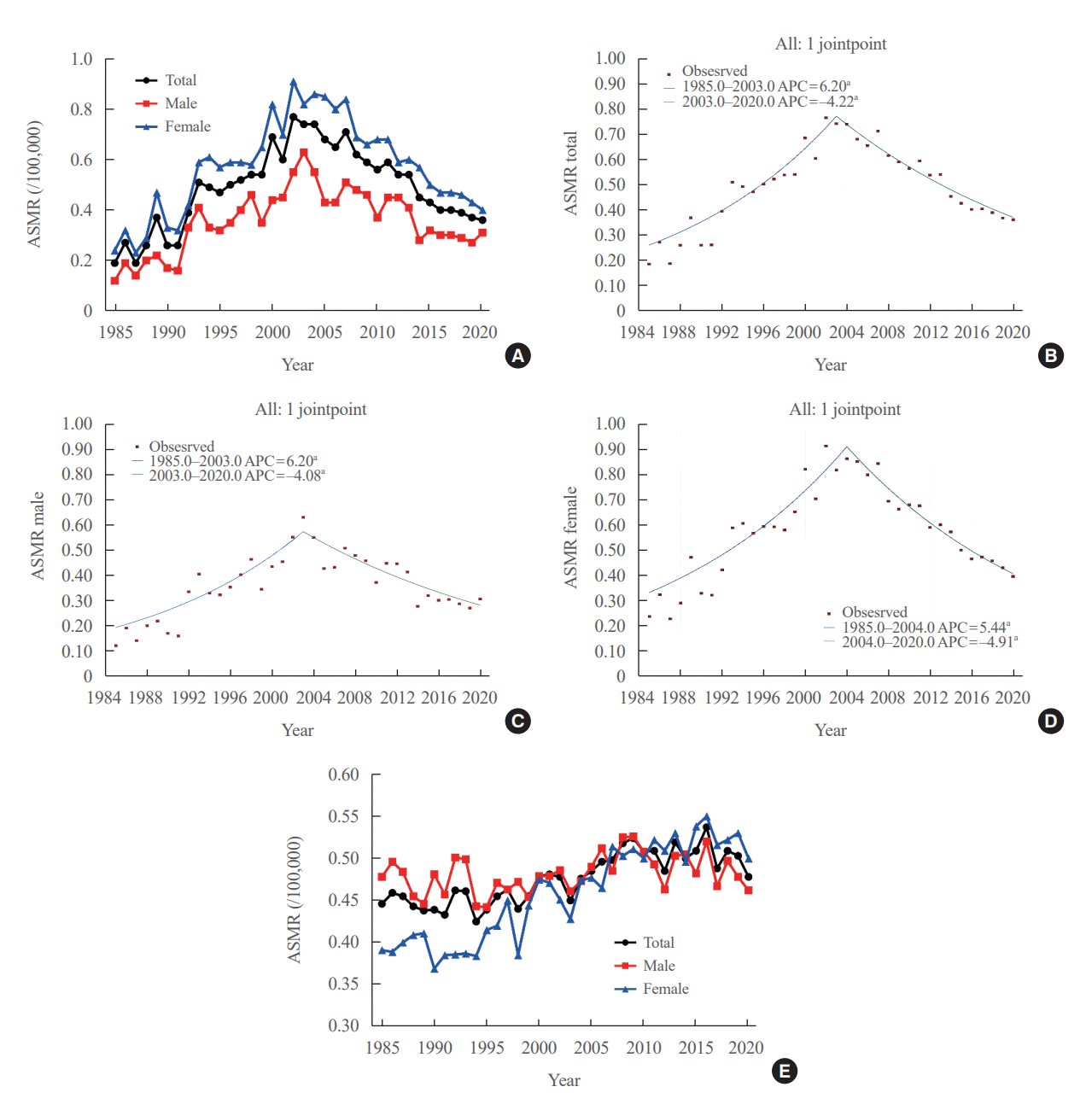
Fig. 2.
(A) Age-standardized mortality rates (ASMR) of thyroid cancer in Korea according to age group. Annual percent change (APC) of ASMR in those (B) <45, (C) 45–54, and (D) ≥55 years of age. (E) Long-term trends in United States ASMR of thyroid cancer according to age in the Surveillance, Epidemiology, and End Results program. aStatistically significant.
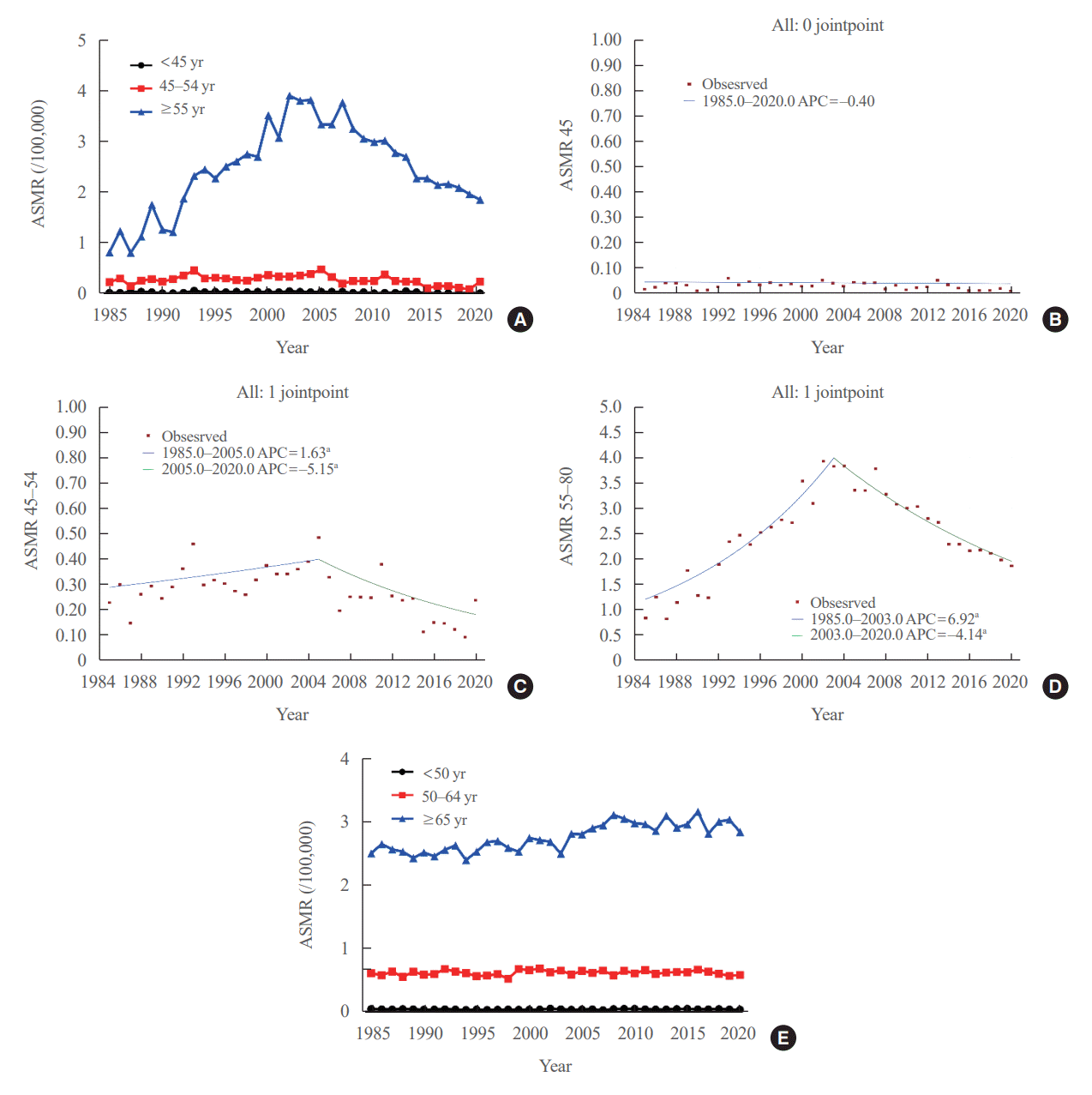
Table 1.
Age-Standardized Mortality Rates (per 100,000) of Thyroid Cancer from 1985 to 2020 in Korea
Table 2.
Trends in Thyroid Cancer Mortality Throughout Korea between 1985 and 2020 Using a Joinpoint Regression Model
|
Trend 1 |
Trend 2 |
AAPC (1985–2020) | ||||
|---|---|---|---|---|---|---|
| Year | APC (95% CI) | Year | APC (95% CI) | |||
| Total | 1985–2003 | 6.204 (5.034 to 7.387)a | 2003–2020 | −4.218 (−5.047 to −3.381)a | 1.008 (0.322 to 1.698) | |
| Men | 1985–2003 | 6.198 (4.401 to 8.026)a | 2003–2020 | −4.084 (−5.299 to −2.853)a | 1.073 (0.035 to 2.122)a | |
| Women | 1985–2004 | 5.439 (4.309 to 6.582)a | 2004–2020 | −4.910 (−5.936 to −3.872)a | 0.576 (−0.163 to 1.32) | |
| Age, yr | ||||||
| <45 | 1985–2020 | −0.403 (−1.592 to 0.799) | −0.403 (−1.592 to 0.799) | |||
| 45–54 | 1985–2005 | 1.630 (0.116 to 3.167)a | 2005–2020 | −5.146 (−8.782 to −1.364) | −1.331 (−3.100 to 0.47) | |
| ≥55 | 1985–2003 | 6.917 (5.647 to 8.203)a | 2003–2020 | −4.136 (−4.936 to −3.329)a | 1.398 (0.682 to 2.119)a | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download