Abstract
Bilateral vertebral artery occlusive disease has been considered as a favorable condition with good collaterals. However, the prognosis of acute ischemic stroke secondary to symptomatic bilateral vertebral artery occlusion (BVAO) and endovascular treatment (EVT) has rarely been reported. We retrospectively selected patients with acute ischemic stroke admitted for symptomatic BVAO between January 2020 and February 2023. All patients with ischemic stroke were evaluated for ischemic lesion and arterial status using brain imaging and angiography. The prognosis of acute stroke with symptomatic BVAO was compared between EVT and conventional treatment. Outcomes were evaluated using modified Rankin Scale (mRS) score at 3 months follow-up. Within the study period, 17 of 2,655 acute ischemic stroke patients were diagnosed with ischemic stroke with symptomatic BVAO. The median age of these patients was 70 (interquartile range 44–89) years, and 13 (76%) were male. Seven patients received emergent EVT with stenting and 10 patients received conventional medical treatment only. Nine of 10 patients with conventional treatment had in-hospital stroke progression and developed new ischemic lesions in the pons and midbrain. Five patients with fetal and hypoplastic posterior communicating artery presented bilateral cerebral peduncular lesions. At 3 months follow-up, 6 patients (35%) had favorable outcomes (mRS 0–2), of which 5 were treated with vertebral artery stenting and 1 received conventional treatment. Ischemic stroke in patients with acute symptomatic BVAO is uncommon. However, stroke progression is common, and the prognosis of most patients is poor. Rescue management such as EVT might be considered for symptomatic BVAO.
Vertebral artery is the most common occlusive site in patients with posterior circulation ischemic stroke [1]. Vertebral artery occlusive disease is attributed to atherosclerosis, and is often accompanied by contralateral vertebral artery stenosis, hypoplasia, or the vertebral artery terminating in posterior inferior cerebellar artery (PICA) [2]. Hypoperfusion and embolism from and to the intracranial vertebral artery (ICVA) have been demonstrated as possible mechanisms of brain ischemia associated with vertebral artery disease [3].
Due to progressive atherosclerotic changes in vertebral artery, bilateral vertebral artery occlusive disease may lead to a well-developed collateral flow and is known to present favorable clinical outcomes [4]. However, the clinical presentation of bilateral vertebral artery occlusion (BVAO) is uncommon compared to that of other intracranial arteries [5]. The prognosis of acute symptomatic BVAO has rarely been reported, with only a few studies [6].
Although the vertebral artery is a common site of stenosis or occlusion, the optimal treatment of vertebral artery disease remains controversial. Several studies have attempted endovascular treatment (EVT) of the vertebral artery to prevent the relapse of ischemic stroke; they reported that EVT was superior to medical treatment alone, with a lower rate of restenosis and complications. However, cases of BVAO were not specified [7].
We studied the clinical course and outcomes of acute ischemic stroke due to BVAO and the prognosis of EVT, such as thrombectomy with ICVA stenting, to improve the perfusion of the posterior circulation.
Data from patients admitted to our tertiary medical center for acute ischemic stroke during the study period were retrospectively analyzed. Symptomatic BVAO was defined as the presence of neurological symptoms associated with posterior circulation stroke along with an ischemic lesion on computed tomography (CT) or magnetic resonance imaging (MRI) that could be attributed to BVAO. BVAO was confirmed by CT angiography (CTA) and MR angiography (MRA). Cases with stenosis or hypoplasia of at least 1 vertebral artery were excluded.
Clinical data were obtained from patients’ medical records, including demographic features, vascular risk factors, time from onset to door, National Institutes of Health Stroke Scale (NIHSS) score during admission and at discharge, modified Rankin Scale (mRS) score at discharge, 2-week, 3-month follow-up, revascularization therapy such as intravenous thrombolysis and EVT, medical treatment, and imaging. The time of stroke onset was recorded if witnessed, or from the last known normal time if the exact time was not known. Stroke severity was assessed using the NIHSS. Imaging data included brain CT, CTA, MRI, MRA, and digital subtraction angiography (DSA) images obtained during the admission period. In our institutional imaging protocol, patients who visited the emergency room initially underwent brain CT, CTA from the aortic arch to the vertex with or without perfusion imaging, and brain MRI and MRA within 24 hours after admission, 5 days after admission, or after neurological deterioration. Collateral flow state was assessed on the basis of initial CTA, MRA, and DSA.
On admission, a stroke neurologist performed a neurological assessment based on the NIHSS score. Inclusion criteria for rescue EVT were as follows: (1) procedure initiated within 24 hours of symptom onset or the last known time the patient was normal, (2) baseline NIHSS score >5, (3) BVAO, (4) no intracranial hemorrhage on brain CT. However, during the study of patients with ischemic stroke due to BVAO, it was found that patients who initially presented with mild stroke symptoms (NIHSS≤5) experienced neurological deterioration during their hospitalization. Therefore, we performed rescue EVT even in cases classified as showing mild stroke symptoms (NIHSS≤5). Patients who presented to the hospital >24 hours after symptom onset were not considered for rescue EVT. Written informed consent for EVT was obtained from each patient’s family. Initially, DSA was performed to evaluate the occlusion sites and the status of the collateral circulation. The choice of EVT procedure was at the discretion of the interventional neurologist. After confirming ease of catheter access to the vessel, the interventional neurologist proceeded with EVT. A stent retriever (SolitaireTM; Medtronic) with simultaneous aspiration (5Fr SofiaTM; MicroVention) was used as the first-line EVT. After thrombectomy, balloon angioplasty (GatewayTM; Stryker) and stent angioplasty (EnterpriseTM2; Cerenovus) were performed. If restenosis or occlusion was detected, tirofiban (0.5–1.0 mg) was injected through the guiding catheter, and additional tirofiban was continuously infused intravenously for 12 hours. After revascularization therapy, the systolic blood pressure was maintained at <180 mmHg. Brain MR and MRA were performed within the first 24 hours after the procedure and 5 days after admission. Antithrombotic agents were initiated after confirmation of hemorrhage or hemorrhagic transformation on brain CT or MRI.
Inclusion criteria of conventional medical treatment were as follows: (1) patients who do not meet the inclusion criteria for rescue EVT and (2) patients who attempted rescue EVT but underwent only DSA due to difficulty in vertebral artery selection. Patients without a cardiogenic embolic source, such as those with atrial fibrillation, were administered dual antiplatelet agents (aspirin 100 mg and clopidogrel 75 mg) with high-intensity statins (atorvastatin 40 mg or rosuvastatin 20 mg). The systolic blood pressure was allowed up to 220 mmHg during the acute phase. Comorbidities such as diabetes mellitus and hyperlipidemia were also treated concomitantly.
The NIHSS score was used to assess neurological outcomes. Neurological improvement was defined as the difference between the discharge NIHSS score and the baseline NIHSS score. Neurological deterioration was defined as an incremental change in total NIHSS score with new ischemic lesions on brain MRI and was assessed using the difference between the severe stage NIHSS score and the baseline NIHSS score. Functional outcome was assessed by mRS at discharge, 2 weeks, and 3 months after discharge. Good clinical outcome was defined as no stroke recurrence and an mRS score ≤2 at 3 months after discharge.
Among the 2,655 patients who were admitted to our hospital due to acute ischemic stroke during study period, 17 patients with symptomatic BVAO were included. The comparison between conventional treatment group and EVT group are shown in Table 1.
Ten patients received conventional treatment. Two of them underwent DSA followed by an attempted EVT, but due to difficulty in selecting vertebral artery orifice, the procedure was terminated, and they received conventional treatment. Dual-antiplatelet agents (aspirin 100 mg and clopidogrel 75 mg) and high-intensity statins (atorvastatin 40 mg or rosuvastatin 20 mg) were initially administered as conventional treatment. The median onset to door time was 49 hours (range 6–144 hours). The median initial NIHSS score was 5 points (range 0–18 points). The median increase in NIHSS score at the most severe stage from admission was 9 points (range 1–16). The median time between admission and the highest NIHSS score was day 9 of hospitalization (range 3–14 days). Nine of the 10 patients who received medical treatment without EVT presented an increase in the NIHSS score with additional ischemic lesions in the pons and/or midbrain (Fig. 1). Five patients presented bilateral peduncular ischemic stroke accompanied by a lesion in the bilateral pons that progressed to locked-in syndrome during hospitalization (Fig. 2). At discharge, 2 patients died due to multi-organ failure associated with systemic infection and cardiogenic shock following acute myocardial ischemia, and 7 patients had severe disability with mRS 4 and mRS 5 (progression to locked-in syndrome). During follow-up, 3 of the 4 patients discharged with mRS 5 died and 1 patient was discharged with an mRS of 4 and had recurrent stroke in the bilateral pons and progressed to mRS 5. Only 1 patient had a favorable clinical outcome with an mRS of 1.
Seven patients initially underwent rescue EVT. The median onset to door time was 9.5 hours (range 5–16 hours). The median initial NIHSS score was 8 points (range 3–17 points). After mechanical thrombectomy, balloon angioplasty and stent placement were performed in the V4 segment of the vertebral artery (Fig. 3). There were no complications, such as intracerebral hemorrhage, hemorrhagic transformation, dissection, or re-occlusion, after the procedure. No patient who received EVT presented with neurological deterioration. The median decrease in NIHSS score at discharge was 5 points (range 2–10 points) compared with that at admission. At the 3 months follow-up, 5 patients presented with favorable clinical outcomes. Of the 2 patients with unfavorable outcomes at 3 months, 1 died of an unknown cause and the other who also had basilar artery occlusion initially presented locked-in syndrome with an mRS of 5.
In this study, we report that the clinical course and outcomes of patients with symptomatic BVAO were mostly unfavorable, and that EVT with vertebral artery stenting may be safe and helpful in preventing neurological deterioration.
A previous study reported that bilateral vertebral artery occlusive disease presented well-developed collateral flows and anastomoses from the occipital artery, posterior communicating artery, PICA, or thyro-cervical trunk and presented favorable outcomes [8]. However, BVAO can cause low flow in the posterior circulation, leading to stepwise stroke progression in the brain stem and cerebellum [9]. Despite intensive medical treatment, patients may experience neurological deterioration and relapse of ischemic stroke due to collateral flows which usually supply insufficient cerebral perfusion in the presence of increased oxygen demand [10]. As shown in Table 1, almost all patients who received conventional treatment presented neurological deterioration during hospitalization due to bilateral ischemic stroke lesions in the pons and/or midbrain, which are well known to have a grave prognosis [11]. Decreased bilateral vertebral artery flow induces neurological deterioration due to inadequate perfusion of the basilar artery through poor collaterals despite medical treatment [12].
Mechanical thrombectomy for EVT is considered to provide blood flow to poorly perfused regions. One study on EVT for posterior circulation stroke reported cases with good outcomes in chronic ischemic stroke patients with intracranial BVAO [13]. In our study, 7 patients received EVT with vertebral artery stenting before stroke progression. All patients were confirmed to have acute BVAO. In all cases, vertebral artery stenosis was confirmed after mechanical thrombectomy, and stent angioplasty was performed. There were no complications after rescue EVT. Almost all patients who received rescue EVT achieved improvement in neurological status with no stroke recurrence and were able to walk independently after discharge, except for 1 who had ischemic stroke lesions in the bilateral pons. Hence, reconstitution of anterograde flow through recanalization of the vertebral artery before neurological deterioration may be helpful to prevent stroke progression.
This study has several limitations. First, the sample size was small (n=17). Only 7 patients underwent EVT with vertebral artery stenting. Therefore, further clinical trials with a larger sample size are required to prove that EVT is safe and optimal for BVAO. Second, this study was based on a retrospective analysis, which could lead to selection bias. Third, regarding confirmation of the collateral status of the patients, only 10 patients received DSA. The other 7 patients had a limitation in confirming collateral flows that filled the basilar artery. Hence, it was difficult to differentiate whether the 7 patients had acute occlusion or chronic occlusion. In addition, there were limitations in evaluating the prognosis based on collateral status in the conventional treatment group. Fourth, during DSA prior to EVT, identifying the vertebral artery orifice can be challenging. EVT may be difficult or impossible to perform when the orifice of the vertebral artery is not visualized.
In conclusion, the prognosis and clinical outcomes of acute symptomatic BVAO are unfavorable due to inadequate perfusion in the caudal pons and midbrain. Uncompensated collateral flow leads to stepwise stroke progression despite conventional medical treatment. Therefore, rescue EVT for reconstitution of anterograde flow through recanalization of the vertebral artery might be considered to prevent neurological deterioration.
Notes
Ethics Statement
Our study has received the Institutional Review Board of the School of Medicine, Chosun University approval (IRB FILE No: CHOSUN 2023-09-008). This article does not include any information that may identify the patient.
Author Contributions
Concept and design: SHA. Analysis and interpretation: BWH, MAL, and SHA. Data collection: BWH, SWH, JHK, and HSK. Writing the article: BWH and SHA. Critical revision of the article: BWH and SHA. Final approval of the article: SHA. Statistical analysis: SHA. Overall responsibility: SHA.
REFERENCES
1. Caplan L. Posterior circulation ischemia: then, now, and tomorrow. The Thomas Willis lecture-2000. Stroke. 2000; 31:2011–2023.

2. Caplan L, Chung CS, Wityk R, Glass T, Tapia J, Pazdera L, et al. New England Medical Center posterior circulation stroke registry: I. Methods, data base, distribution of brain lesions, stroke mechanisms, and outcomes. J Clin Neurol. 2005; 1:14–30.

3. Caplan LR. The intracranial vertebral artery: a neglected species. The Johann Jacob Wepfer Award 2012. Cerebrovasc Dis. 2012; 34:20–30.

4. Shin HK, Yoo KM, Chang HM, Caplan LR. Bilateral intracranial vertebral artery disease in the New England Medical Center, posterior circulation registry. Arch Neurol. 1999; 56:1353–1358.

5. Wityk RJ, Chang HM, Rosengart A, Han WC, DeWitt LD, Pessin MS, et al. Proximal extracranial vertebral artery disease in the New England Medical Center posterior circulation registry. Arch Neurol. 1998; 55:470–478.

6. Rüegg S, Engelter S, Jeanneret C, Hetzel A, Probst A, Steck AJ, et al. Bilateral vertebral artery occlusion resulting from giant cell arteritis: report of 3 cases and review of the literature. Medicine (Baltimore). 2003; 82:1–12.

7. Maitas O, Bob-Manuel T, Price J, Noor A, Obi K, Okoh N, et al. Vertebral artery interventions: a comprehensive updated review. Curr Cardiol Rev. 2023; 19:e170322202296.

8. Bogousslavsky J, Gates PC, Fox AJ, Barnett HJ. Bilateral occlusion of vertebral artery: clinical patterns and long-term prognosis. Neurology. 1986; 36:1309–1315.

9. Ortiz de Mendivil A, Alcalá-Galiano A, Ochoa M, Salvador E, Millán JM. Brainstem stroke: anatomy, clinical and radiological findings. Semin Ultrasound CT MR. 2013; 34:131–141.

10. Duan H, Chen L, Shen S, Zhang Y, Li C, Yi Z, et al. Staged endovascular treatment for symptomatic occlusion originating from the intracranial vertebral arteries in the early non-acute stage. Front Neurol. 2021; 12:673367.

12. Tariq N, Maud A, Shah QA, Suri MF, Qureshi AI. Clinical outcome of patients with acute posterior circulation stroke and bilateral vertebral artery occlusion. J Vasc Interv Neurol. 2011; 4:9–14.
Fig. 1.
Initial brain magnetic resonance angiography (MRA) and time course of diffusion-weighted imaging of patient No. 9. (A) Brain MRA showed bilateral vertebral artery occlusion. (B) Initial diffusion-weighted imaging showed ischemic lesions in right cerebellum, bilateral pons, and right midbrain. (C) Five-day diffusion-weighted imaging showed enlargement of lesions in pons and midbrain. (D) Twelve-day diffusion-weighted imaging showed new ischemic lesions in medial pons and bilateral cerebral peduncles in midbrain.
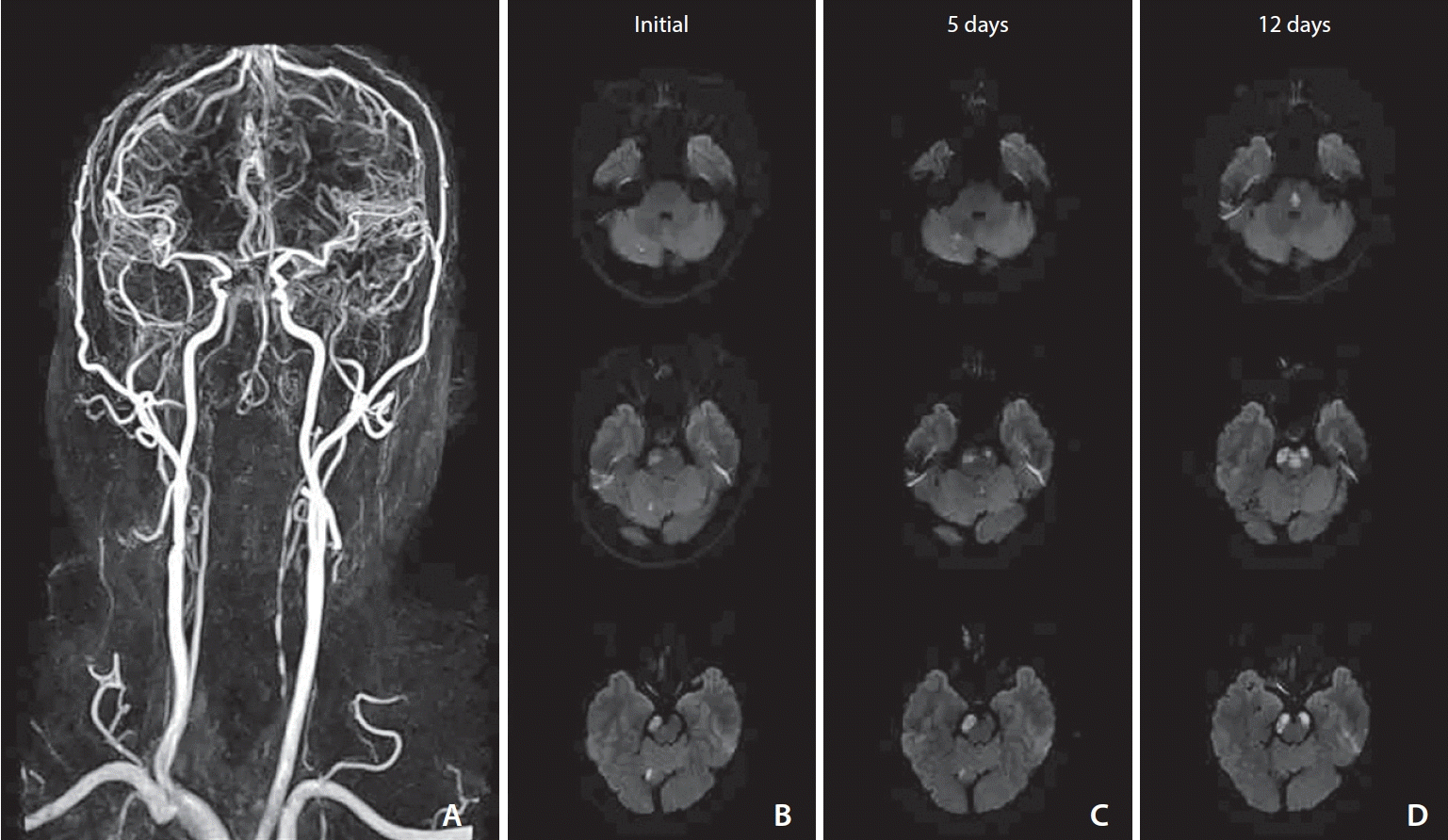
Fig. 2.
Lesion tomography of ischemic stroke caused by bilateral vertebral artery occlusion patients who received medical treatment alone. Five patients (No. 2, 3, 4, 6, 9) presented ischemic lesions in medial pons and bilateral cerebral peduncles.
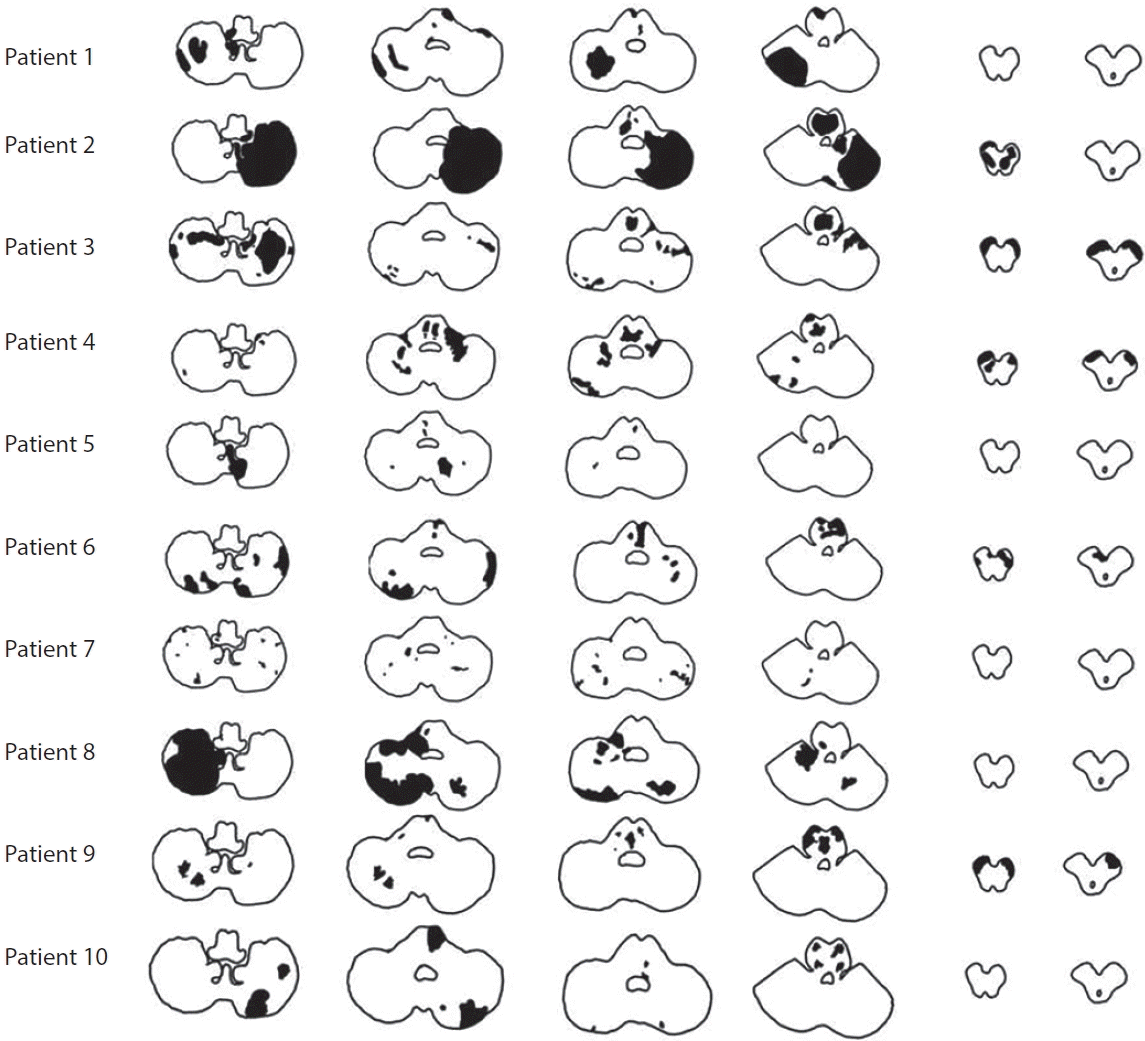
Fig. 3.
Rescue endovascular treatment of patient No. 16. (A, B) Right and left internal carotid artery angiography revealed no collateral flows filling the basilar artery. (C) Left vertebral angiography showed occlusion of left vertebral artery (V4 segment). (D) Using a stent retriever (4×20 mm, SolitaireTM; Medtronic), mechanical thrombectomy was performed. (E) Left vertebral artery angiography showed severe stenosis of the left vertebral artery (arrow). (F, G) Balloon angioplasty (3.0×15 mm, GatewayTM; Stryker) (6 and 10 atm) and stenting (4×30 mm, EnterpriseTM2; Cerenovus) (circles) of the left vertebral artery (V4 segment) were performed. (H) Tirofiban (1.0 mg) was injected through the guiding catheter due to re-stenosis of left vertebral artery. (I) Red-colored specimens were retrieved.
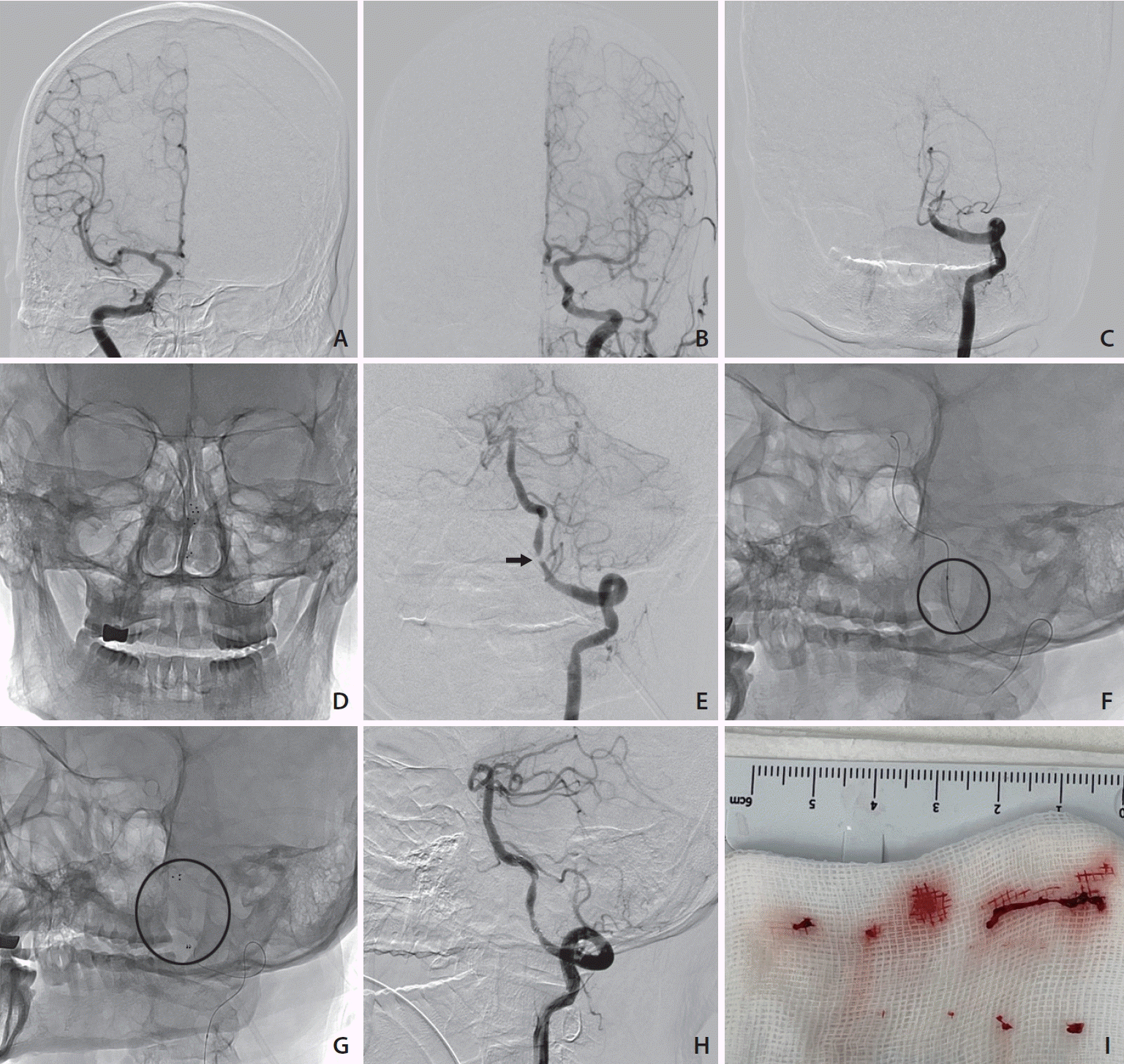
Table 1.
Comparison between rescue EVT group and intensive medical treatment group




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download