INTRODUCTION
MATERIALS AND METHODS
RESULTS
Table 1.
Data are mean±standard deviation, number (%), or median (interquartile range).
NIHSS, National Institutes of Health Stroke Scale; ND, neurologic deterioration; ASPECT, Alberta Stroke Program Early computed tomography Score; MCA, middle cerebral artery; ICA, internal carotid artery; BA, basilar artery.
Table 2.
| Total patients (n=26) | Good outcome patients (n=18) | Poor outcome patients (n=8) | P-value | ||
|---|---|---|---|---|---|
| Mean LKNT to arterial puncture time (h) | 59.9±38.5 | 58.1±26.6 | 63.9±61.4 | 0.726 | |
| Mean onset to hospitalization time (h) | 41.1±27.1 | 41.3±25.2 | 40.8±32.8 | 0.965 | |
| Mean onset to ND time (h) | 63.2±41.5 | 58.5±26.2 | 87.8±96.2 | 0.274 | |
| Only thrombectomy | 4 (15.3) | 0 (0) | 4 (50.0) | 0.001 | |
| Thrombectomy with angioplasty* | 12 (46.1) | 10 (55.5) | 2 (25.0) | 0.149 | |
| Thrombectomy with ballooning | 1 (8.3) | 1 (5.5) | 0 (0) | 0.516 | |
| Thrombectomy with stenting | 5 (41.6) | 3 (16.6) | 2 (25.0) | 0.635 | |
| Thrombectomy with ballooning and stenting | 6 (50.0) | 6 (33.3) | 0 (0) | 0.067 | |
| Only angioplasty | 9 (34.6) | 8 (44.4) | 1 (12.5) | 0.114 | |
| Ballooning | 0 (0) | NA | NA | NA | |
| Stenting | 2 (22.2) | 1 (5.5) | 1 (12.5) | 0.558 | |
| Ballooning and stenting | 7 (77.7) | 7 (38.8) | 0 (0) | 0.040 | |
| Stent insertion | 20 (76.9) | 17 (94.4) | 3 (37.5) | 0.001 | |
| Intracranial stent insertion | 11 (42.3) | 8 (44.4) | 3 (37.5) | 0.741 | |
| Carotid stent insertion | 9 (34.6) | 9 (50.0) | 0 (0) | 0.013 | |
Table 3.
| Total patients (n=26) | Good outcome patients (n=18) | Poor outcome patients (n=8) | P-value | |
|---|---|---|---|---|
| Final TICI grade (≥2b or 3) | 24 (92.3) | 18 (100) | 6 (75.0) | 0.027 |
| Hemorrhagic infarction type | 3 (11.5) | 1 (5.5) | 2 (25.0) | 0.152 |
| Parenchymal hematoma | 1 (3.8) | 1 (5.5) | 0 (0) | 0.497 |
| Subarachnoid hemorrhage | 4 (15.3) | 1 (5.5) | 3 (37.5) | 0.037 |
| Symptomatic ICH | 0 (0) | 0 (0) | 0 (0) | |
| Mortality | 2 (7.6) | 0 (0) | 2 (25.0) | 0.027 |
| Any other adverse events* | 0 (0) | 0 (0) | 0 (0) |
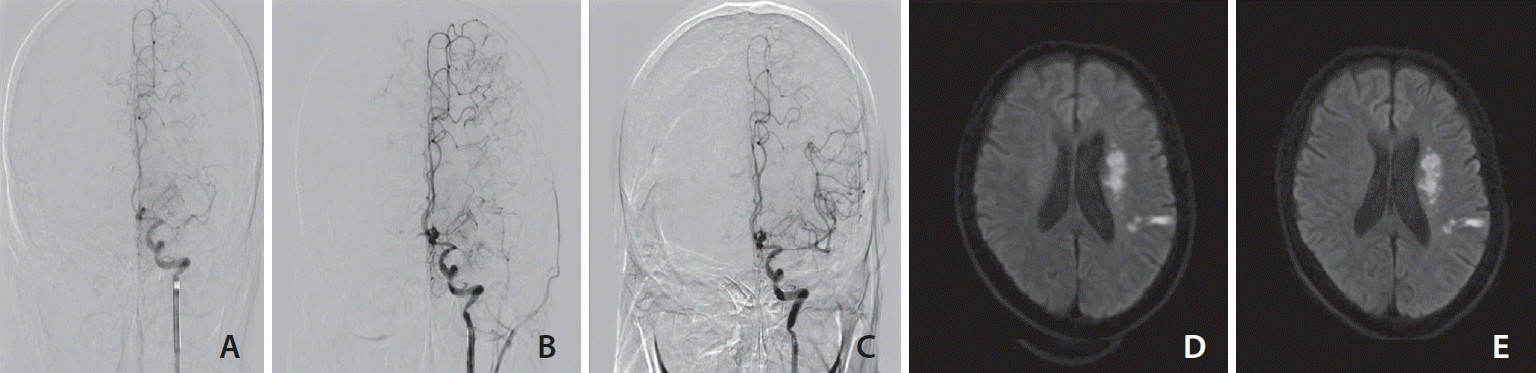 | Fig. 1.Endovascular recanalization therapy and brain diffusion-weighted imaging before and after 24 hours of the procedure. (A) In digital subtraction angiography performed 83.9 hours after occurrence, left MCA proximal site occlusion and decreased filling on distal flow. (B) After 1 thrombectomy, a partially recanalized, but left MCA mid portion diffuse severe atherosclerotic stenosis and decreased distal flow were detected. (C) Stent insertion was carried out, full recanalization and TICI grade 3 of distal flow were identified. (D) Diffusion restriction of left periventricular area and temporo-parietal lobe at initial. (E) After 24 hours of the procedure, there was no significant difference compared with the previous ischemic lesion. MCA, middle cerebral artery; TICI, thrombolysis in cerebral infarction. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download