This article has been
cited by other articles in ScienceCentral.
Abstract
We present a case of delayed migration of an open-cell design carotid stent, which is a rare complication following carotid artery stenting (CAS). A 65-year-old patient with carotid artery stenosis underwent CAS with an open-cell stent, initially achieving successful deployment. However, 4 months later, the stent migrated and resulted in restenosis. The patient underwent balloon angioplasty and received an additional stent, leading to improved blood flow. The rarity of stent migration, particularly in the absence of risk factors, highlights the need for clinicians to be vigilant and consider early imaging follow-up for patients at risk of this complication after CAS.
Go to :

Keywords: Carotid stenosis, Stents, Angioplasty, Complication
INTRODUCTION
Carotid artery stenting (CAS) has been used as an alternative to carotid endarterectomy (CEA) for selected patients with carotid artery stenosis at high surgical risk [
1]. Previous studies have reported various complications associated with CAS, including stroke, intracranial hemorrhage, in-stent stenosis, and occlusion, complicating folding deformity [
2,
3]. Especially, delayed migration of an open-cell design carotid stent is a very rare complication [
4-
7]. Herein, we present a case of delayed migration of a tapered opencell design carotid stent.
Go to :

CASE REPORT
A 65-year-old male presented to the hospital with intermittent left arm weakness. The patient had a medical history of tobacco use, coronary artery disease, dyslipidemia, and hypothyroidism. He was on medical treatment with daily doses of aspirin 100 mg, atorvastatin 10 mg, ezetimibe 10 mg, and levothyroxine 0.1 mg. Brain computed tomography angiography (CTA) revealed a significant stenosis of the right proximal internal carotid artery (ICA) (
Fig. 1A,
B) and magnetic resonance imaging (MRI) showed perfusion delay in the relevant arterial territory without evidence of acute cerebral infarction. Because of his recurrent ischemic symptoms, carotid stenting was planned and clopidogrel 75 mg daily was started 10 days before admission.
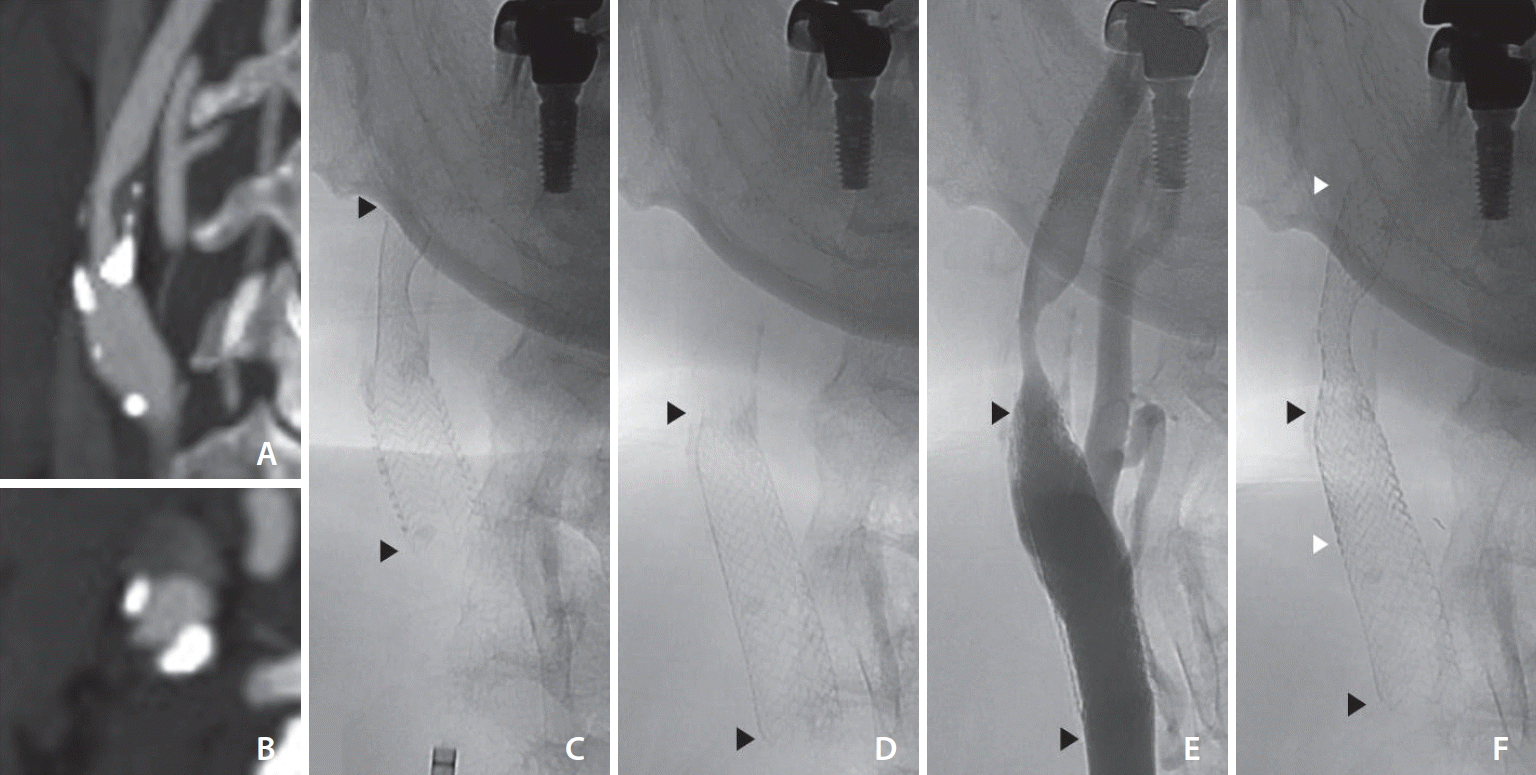 | Fig. 1.(A, B) Brain computed tomography angiography showing severe stenosis with calcified plaque. Fluoroscopic images showing (C) the initial stent insertion, (D) downward migration of the stent on 4 months follow-up, (E) restenosis distal to the stent, and (F) after the second stent insertion. Arrowheads point to both ends of the initial stent (black) and the second stent (white). 
|
Under local anesthesia with 1% lidocaine
via the right common femoral artery, a 90 cm long 8F Fubuki guiding catheter (Asahi Intec) was placed in the right common carotid artery. The stenosis of the right proximal ICA was measured to be 15 mm in length with 60% stenosis according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria. The gradient of vessel diameter was not large with a proximal diameter of 8.4 mm and a distal diameter of 6.1 mm. After the placement of the Emboshield NAV6 (Abbott Vascular), an Acculink RX tapered stent (7–10×40 mm; Abbott Vascular) was deployed across the lesion with sufficient proximal and distal margins (
Fig. 1C). Poststent balloon angioplasty was not performed due to adequate stent lumen. Control angiography showed patent stented segment of the right ICA with adequate stent apposition to the vessel wall. The patient was discharged home 2 days later with no neurological deficits and continued dual antiplatelet therapy.
Four months after discharge, the patient presented to our hospital with a complaint of right facial (parotid area) pain, tenderness and swelling for 5 days. Neck CT with contrast suggested parotitis and downward migration of the stent. After a few days, the patient complained a foreign body sensation in the right neck. Follow-up brain CTA and MRI showed downward migration of the stent with significant stenosis at the previous lesion of the right ICA stenosis and perfusion delay in the relevant arterial territory.
One week after the follow-up MRI, the patient was readmitted for management of carotid restenosis. The right carotid angiogram confirmed stent migration and restenosis of the previous stenotic lesion with 70% stenosis (
Fig. 1D,
E). Compared to the previous angiogram, the stent had migrated approximately 14 mm inferiorly. Pre-stenting balloon angioplasty was performed with a 4 mm×40 mm Aviator Plus balloon (Cordis). An 8 mm×40 mm Precise RX stent (Cordis) was successfully deployed across the stenotic lesion with the Emboshield NAV6. The stent was deployed overlapping the distal half of the previously delivered stent (
Fig. 1F). The control angiogram showed a spanned stenotic carotid segment with improved antegrade flow in the right ICA. The patient was discharged home 2 days after the procedure with no neurological complications. Since then, the patient did not complain of any signs or symptoms, and no further studies were performed.
Go to :

DISCUSSION
Stent migration after CAS is a rare complication but has been reported to occur infrequently depending on the patient’s lesion and associated carotid arteries [
8,
9]. Excessive vessel tortuosity or the formation of dense fibrosis after CEA may increase the risk of stent migration. Some authors reported that stent migration was observed when the lesion had severe stenosis with excessive kinking of >90 degrees at the distal end or when the lesion had a previous CEA [
10,
11]. However, in our case, the stenotic vessel had a calcified lesion without tortuosity, and an open-cell stent was used with a length and diameter sufficient to cover the stenotic lesion and approximate the normal arterial wall.
Interestingly, stent migration does not appear to be affected by stent design, such as open-cell [
7,
10] or closed-cell [
4,
6]. Closed-cell stent migration may result from the watermelon-seeding effect, where the stent moves toward the larger proximal vessel due to diameter differences [
6]. In contrast, open-cell stents offer flexibility and resistance to deformation, allowing independent segment expansion even in vessels with diameter variations. In addition, open-cell nitinol stents are generally less prone to migration. In this case, the diameter difference between the two ends of the vessel was only 2.3 mm, and despite the use of an oversized tapered open-cell nitinol stent, stent migration occurred.
In published cases, stent migration was observed not only during follow-up, as in our case, but also immediately after CAS (
Table 1) [
4-
7,
10]. In general, endothelial growth after stenting integrates the stent into the vessel wall and prevents migration. Within a few weeks, the stent struts are expected to be integrated into the vessel wall, making migration or dislodgement difficult. Therefore, stent migration, if it occurs, is likely to have occurred within a few days after CAS.
Table 1.
Summary of case reports for the stent migration
|
Ref No. |
Authors (y) |
Sex/age |
Carotid artery stent
|
Stent migration
|
Retreatment |
Follow-up |
|
Indication |
Stent |
Duration/study f/u |
Finding |
Related factor |
|
4 |
Castriota et al. 2008 |
M/69 |
Severe (95%) asymptomatic, right. |
SES, overlapped by Wallstent (nontapered closed-cell) |
15 mo/CDU |
Distal migration |
Tortuosity distal to stenosis. |
7.0×30 mm Carotid Wallstent |
Stable on 8 mo, 10 mo, and 13 mo CDU |
|
5 |
Yan et al. 2009 |
M/5 |
Williams syndrome. |
BES (Genesis), nontapered |
7 mo/DSA |
Distal migration of BES to the distal CCA |
|
None |
Stable for 5 y |
|
Symptomatic stenosis in right brachiocephalic artery (90%) and left CCA (60%). |
|
6 |
Badruddin et al. 2011 |
M/70 |
Severe symptomatic (TIA) stenosis (99%), left. |
SES, Xact, 7×20 mm, nontapered closed-cell |
8 mo/CTA |
Proximal downward migration |
Relatively small sized and short stent. |
Tapered 6–8×40 mm Xact stent |
f/u |
|
Calcified stenotic lesion. |
|
7 |
Endo et al. 2023 |
M/70 |
Restenosis (80%) 6 months after CEA for symptomatic right ICA stenosis (86%) due to radiotherapy. |
SES, tapered open-cell stent (Protégé RX 10/7×40 mm) |
#2 d/CTA |
Proximal migration into CCA |
Large difference between proximal (7.4 mm) and distal (2.8 mm) vessel diameters. |
None |
Stable on 6 mo DSA f/u |
|
Post-RTx and endarterectomy (patch graft) state. |
Stable for 3 y |
|
10 |
Swarnkar et al. 2014 |
M/63 |
Restenosis after CEA, bilaterally. |
SES, nontapered, overlapping stents |
Right: #4 d/DSA. |
Right: caudal migration of proximal stent and cranial migration of distal stent (9 mm gap) |
Bilateral carotid CEA complicated by hematoma formation. |
Right: during procedure; additional stent (Acculink 6×20 mm) |
#5 d |
|
Right: 90% stenosis with 5.2 cm in length. |
Right: Acculink 5×40 mm and 5×20 mm |
Left: #2 d/CDU |
Fibrosis and loss of elasticity at the surgical site. |
Right: stable |
|
Left: 80% stenosis with 7 cm in length. |
Left (#4 days after right): Acculink 5×40 mm and 6×40 mm |
Left: 12 mm separation |
#4 days after stent: 2nd additional stent (Acculink 6×40 mm) |
Left: observation for migration |

In summary, we have demonstrated the rare case of delayed migration of a tapered open-cell nitinol stent in the absence of risk factors for stent migration. Clinicians should be aware of this rare complication after CAS and early follow-up with noninvasive imaging should be considered if the lesion is at risk for stent migration.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download