Abstract
Objectives
Concha cartilage is recommended for correction of cleft nasal deformities. Morbidities at the donor site have been reported in esthetic rhinoplasty cases. Reports on cleft patients are limited, so we investigated the complications of concha cartilage harvesting using the retroauricular approach in cleft rhinoplasty and their management.
Materials and Methods
This was a retrospective review of the charts of 63 patients with cleft deformities who underwent septorhinoplasty with concha cartilage. All cases were harvested using a retroauricular approach. Data on patient demographics, surgery type, amount of cartilage harvested, and complications were gathered.
Cleft lip and palate (CLP) are common deformities in the oral and maxillofacial regions. Cleft lip (CL) is found with or without cleft palate (CP) in 1 in 1,000 newborns1. The incidence of isolated CP is approximately 1 in 2,500. CLP is becoming more common among Asians than among Caucasians and Africans. Children born with CLP can experience functional and esthetic problems throughout life if treatment is not completed at an appropriate early age. Cleft nasal deformity is one of the deformities observed in patients with CLP. In addition to facial aesthetic issues, CLP patients also have difficulty breathing2. Septorhinoplasty is recommended for cleft nasal deformities. Secondary rhinoplasty is one of the most challenging surgical procedures due to the severe anatomical distortions and scarring from previous surgeries3-5.
Various materials are used to reconstruct the nasal form among patients with clefts, such as autologous cartilage, silicone implants6, and expanded polytetrafluoroethylene7. Polymer-based alloplastic material is associated with a higher rate of complications. Additionally, postoperative infection and material extrusion have been documented8. In contrast, surgeons favor autologous cartilage grafts for cleft rhinoplasty because their use is associated with fewer postoperative complications. Concha cartilage, septal cartilage, and costal cartilage are all used for cleft rhinoplasty. Concha cartilage has several advantages over the other choices: it is easy to harvest and sculpt and can provide enough quantity to reconstruct cleft nasal deformities9-12. The absence of visible scars at the donor site has been cited with all approaches, especially when using a retroauricular approach13. Although the concha cartilage is widely used, complications at the donor site have not been extensively reported, particularly with respect to cleft rhinoplasty.
This study aimed to report the donor site complications associated with a retroauricular approach for harvesting concha cartilage in cleft rhinoplasty.
The institutional review board of Faculty of Dentistry, Mahidol University reviewed and approved the study (COA.No.MU-DT/PY-IRB 2022/051.0310). Medical records between December 2013 and April 2022 at our university were reviewed. The inclusion criteria were patients with CLP who underwent septorhinoplasty with concha cartilage harvesting using a retroauricular approach. Cases were followed at 1 and 2 week(s); 1, 3, 6, and 9 month(s); and 1 year postoperatively. Patients without detailed follow-up data were excluded from the study. Data of interest were demographics (sex, age, and type of cleft), time of rhinoplasty (first or revised case), medical problems, and intraoperative antibiotic.
All patients underwent septorhinoplasty using concha cartilage under general anesthesia. All septorhinoplasty cases were performed by one surgeon (K.B.). The face and selected ear of each patient were exposed and disinfected according to the routine procedure. The concha cartilage was harvested according to the following protocol (Fig. 1): local anesthetic with 2% mepivacaine hydrochloride with epinephrine 1:100,000 (Septodont) was added to provide hemorrhagic control along the incision line and the anterior surface of the concha bowl. The assistant retracted the ear (forward) while the principal surgeon performed the harvest. First, a 3- to 4-cm, curved incision was made using a No. 15 blade. Subperichondrial dissection was then performed at the posterior surface of the concha cartilage using sharp, curved scissors. Three short, 30-gauge hypodermic needles were used to set the boundaries of the harvesting area. These needles can help limit the amount and avoid damage to the antihelix.
A No. 15 blade was used to cut the superior and anterior edges while harvesting the cartilage to avoid injuring the anterior skin of the ear. The anterior surface of the concha cartilage was prepared subperichondrially. The margin was cut and the concha cartilage was harvested. Hemostasis was achieved using bipolar electrocautery. The subcuticular suture was performed with 4-0 polypropylene (Prolene 4-0; Ethicon). A Penrose drain was inserted through the incision line. Bolster gauze, rolled gauze soaked with Vaseline (Unilever), was packed at the anterior surface and sutured with 3-0 nylon (Ethilon 3-0; Ethicon). Then, septorhinoplasty was performed.
The Penrose drain was removed 1 day postoperatively, and the pressure dressing was removed the following day. The bolster gauze was retained for 1 week and removed during a follow-up visit. The suture was removed at 2 weeks postoperatively. The wound at the donor site of all patients was examined at 1, 2, and 4 week(s) and until there were no complaints from the patient.
Sixty-three patients with cleft (21 males, 42 females) who underwent septorhinoplasty using concha cartilage were included. The mean age of the patients was 20.2±5.9 years (range, 13-38 years). Among these patients, 16 presented with a right unilateral cleft, 31 presented with left unilateral cleft, and 16 presented with a bilateral cleft. The left concha cartilage was used in 20 cases, while the right concha cartilage was used in 39. Four cases received rhinoplasty with bilateral concha cartilage. Fifty-four cases underwent secondary rhinoplasty, while the others underwent revision rhinoplasty. Concha cartilage was used concomitantly with septal cartilage in most cases (n=50, 79.4%), while it was used alone for nasal reconstruction in the remaining 13 cases. The antibiotics used intraoperatively and during hospital admission were cefazolin or amoxicillin with clavulanic acid. Clindamycin was used in patients with an allergy to penicillin. The average size of the concha cartilage was 1.48 cm (width) by 2.10 cm (length). Demographic data are presented in Table 1.
Six patients (9.5%) experienced complications at the donor site during the follow-up period. Three cases of delayed wound healing consisting of wound dehiscence and inflammation were recorded (4.8%). Prolonged pain after surgery was found in one case (1.6%) 3 months postoperatively; however, the pain was mild. Postoperative paresthesia was found in one case (1.6%). Prominauris was detected in one patient (1.6%) 6 months postoperatively. Table 2 presents the list of complications documented in the present study compared to previous studies.
Two patients presented wound dehiscence; the first instance was resutured under local anesthesia 1 week postoperatively, while the other was left without surgical intervention. Both cases received cefazolin as an intraoperative antibiotic, and both healed completely. A photograph of a patient with wound dehiscence is shown in Fig. 2. Wound inflammation and postoperative paresthesia healed spontaneously without surgical intervention. Prominauris was noted in one case; however, the patient denied surgical correction. A photograph of the patient with prominauris is shown in Fig. 3. There were no cases of postoperative infection, keloid, or cauliflower-like ear deformities.
Costal cartilage, septal cartilage, and concha cartilage are used frequently for cleft rhinoplasty. Although costal cartilage is the preferred source of graft material for cleft rhinoplasty due to the adequate amount of graft material, a meta-analysis of complications associated with costal cartilage harvesting revealed warping and hypertrophic chest scarring14, chondritis, and cartilage necrosis15,16. Another study reported a case of premature calcification of the costal cartilage in a child17. These demonstrate possible limitations when using costal cartilage.
Septal cartilage is also popular among surgeons due to low donor site morbidity as it is drawn from the same operative field. Furthermore, the quality of septal cartilage is good for use as a columellar strut graft in unequal columellar cases18,19. However, more than 45% of the inferior portion of the caudal strut must be preserved to stabilize the septum20. Additionally, success with septal cartilage is limited in cases of revision rhinoplasty. For example, in patients with cleft, the structure of the septal cartilage is distorted and malformed; as such, septal cartilage alone is not adequate for secondary cleft rhinoplasty. The concha cartilage is used along with septal cartilage to increase the quantity of grafting material. The texture of the concha cartilage is elastic enough to neatly contour the lower lateral cartilage and the alar rim13. Moreover, its curve is similar to that of the lower lateral cartilage. Therefore, concha cartilage can be used when shaving the nasal cartilage. Some grafts are stable enough to support the infrastructure of the nasal cartilage and produce good results21. Importantly, the harvesting technique can be performed by less experienced surgeons, and intraoperative complications are not serious.
Conchal cartilage is popular among surgeons for nasal reconstruction of a cleft nasal deformity. Its advantages are as follows: (1) relatively easy harvesting technique(s), (2) fewer surgical scars, (3) a short operative time, and (4) relatively few or no serious complications. Two techniques exist for harvesting the concha cartilage: the anterior approach22,23 and the posterior or retroauricular approach10,24-26. Of these, the anterior approach is easier because it allows direct visualization of all anatomical boundaries. However, the surgical scar from the anterior approach is more difficult to conceal than that from the retroauricular approach. Although the retroauricular approach is more difficult and has more possible complications, it is preferred among cleft surgeons for esthetic reasons.
Our surgical technique uses local anesthesia for hemostasis and involves hydrodissecting the subperichondrium plane before placing the incision line. Dissection in the proper plane can help to reduce intraoperative muscle bleeding resulting in a postoperative hematoma. The use of a hypodermic needle before cartilage harvesting can control the cartilage outline to prevent collapse of the ear. A unilateral cartilage graft might be inadequate in cases of severe cleft deformity, so bilateral conchal cartilage harvesting can be performed27.
Complications from concha cartilage harvesting among patients with cleft have not previously been reported, and there is no consensus on the best harvesting technique(s). Cauliflower-ear, which is a deformity that occurs because of fibrosis resulting from postoperative hematoma, is common28,29. In our retrospective study, there were no cases of postoperative hematoma.
Three key strategies of our harvesting technique may be summarized as follows. First, a Penrose drain was inserted into the wound and removed 1 day postoperatively. After suturing, the Penrose drain can serve as a means of draining accumulated blood. Second, a bolster dressing was sutured after the procedure and left in place for 1 week. The bolster acts as a pressure device, aiding in hemostasis and facilitating re-adaptation of the wound bed. Finally, hemostasis was performed before suturing, especially at the posterior margin of the donor site defect due to the rich vascular supply. The postauricular skin subdermal layer has perforating and non-perforating branches of the posterior auricular artery30, and these small blood vessels are a possible cause of bleeding and postoperative hematoma.
In 2017, Lan et al.31 reported 5 cases of postoperative hematoma, with no clear predilection of sex. The high frequency of hematoma in their study was possibly due to their concern about pressure necrosis; therefore, there should be a limit on the duration of pressure dressing usage. Pressure necrosis is caused by an ischemic injury to the epidermis, resulting in skin discoloration31. In contrast, our study used only a retroauricular approach, so skin discoloration was not an issue. In 2021, Du et al.26 reported using the simple dressing-fixation method following cartilage harvest and obtained a result similar to ours, with a possible complication from the bolster dressing. One patient presented with a concha bowl defect from pressure necrosis due to prolonged pressure from the bolster dressing.
Consequently, use of bolster or other pressure dressings should be limited to 1 week during the postoperative period32. In 2012, Kim et al.33 compared conventional tie-over dressings after an anterior approach to harvesting concha cartilage and used a Merocel dressing without pressure. These dressings led to fewer complications related to postoperative hematoma and partial skin loss33. Semi-occlusive Merocel dressings provide adequate wound support without excess pressure on the donor site33, although they are not the gold-standard dressing for this situation.
Delayed wound healing was found in three cases in our study. Therefore, use of a mask and glasses should be avoided if possible, as two cases presented with wound dehiscence 2 weeks postoperatively. Treatment in 1 case was resutured with a simple interrupted suture under local anesthesia. Another, due to its small size, did not require treatment. All dehiscence wounds were completely healed by 4 weeks after surgery. In 1991, Jovanovic and Berghaus24 reported 1 case of wound dehiscence. These authors suspected that the cause was the mattress suture fixing the pressure dressing of the spherical sponge, causing necrosis of the skin. They recommended not leaving a mattress suture in for more than 4 days24. In comparison, in our study, a bolster dressing was retained for 1 week to prevent postoperative hematoma. Grobbelaar et al.34 reported the same complication of delayed wound healing after using the retroauricular approach. These authors recommended not to pull the ear while dressing and to avoid sleeping on that side after removing the skin sutures34. One case presented with prolonged wound inflammation: redness was observed on the surrounding skin, and the patient reported discomfort after wearing a mask. However, the wound healed completely without any problems by 2 weeks postoperatively.
In addition, 1 case stated postoperative pain at 3 months. During the coronavirus disease 19 pandemic, it was especially crucial that wound-healing problems were assessed due to prolonged mask-wearing. Patients reported increased pain, discomfort, and wound irritation during this period, which might have been avoided by wearing a mask with a head strap.
Keloid and hypertrophic scars did not occur in our study. In 2017, Lan et al.31 reported 4 cases of keloid. Surgical excision with steroid injection was preferred in 3 cases and there was residual keloid in 2 cases. One patient refused treatment. A slight male predilection was found in the keloid group. Therefore, sex might be a factor in the formation of keloids. Interestingly, all cases with keloids in their study had been treated using a posterior approach31. In contrast, our study did not report any keloids. In 2008, Nemoto et al.25 retrospectively reviewed complications: 2 cases of keloid were found at 1 and 4 months after surgery, respectively. Surgical excision was performed, but keloid recurrence occurred 17 and 12 years after the treatment, respectively25. A curved incision was used in our technique, which helped to reduce wound contraction during the healing period. Furthermore, a subcuticular suture can limit the numbers of keloids and hypertrophic scars, as skin sutures are more likely to cause irritation and inflammation compared to subdermal sutures35. Using a scrupulous surgical technique, iatrogenic keloid scarring can be minimized.
Paresthesia was not mentioned in any studies except ours. One patient complained of postoperative paresthesia 1 month postoperatively. The feeling of numbness was not extensive and resolved by 2 months postoperatively. Unfortunately, the area of numbness was not delineated, so the specific area of the symptoms cannot be summarized. The cranial and spinal nerves supply the auricle. The concha receives innervation from the nervus intermedius, the auricular branch of the vagus nerve, and the greater auricular nerve. The area superior to the concha is innervated by the auriculotemporal and lesser occipital nerves, while the great auricular nerve supplies the inferolateral part of the concha to the lobule36. Based on a retroauricular approach to the concha cartilage, the distal branches of the great auricular nerve and the lesser occipital nerve could be injured; however, the symptoms would be temporary if the main nerve trunk is not injured. The anterior approach achieves better preservation of the sensory nerve as it is distributed along the posterior surface of the ear. Accordingly, an anterior approach might be selected in such patients.
In 1 case, prominauris (or protruding ear) was observed by the examining physician. Correction of this complication after cartilage harvesting depends on a discussion between the patient and surgeon. In the present study, this mild deformity was not recognized as the patient did not complain, not realizing that the problem was abnormal. In 1997, Grobbelaar et al.34 reported 1 case of ear flattening surgery. In 2010, Deleyiannis37 reported 1 case of decreased depth and projection of the conchal bowl, resulting in hearing loss at high frequencies. The conchal bowl was reconstructed with costal cartilage. Changes in conchal dimensions can affect the characteristics of the sound reaching the middle ear37. These problems can be avoided by maintaining an adequate amount of residual cartilage. Adams recommended leaving an at least 2 mm superior outer rim of the concha to prevent ear cartilage from collapsing. At least 3 mm of the external auditory meatus rim was proposed to retain the normal form of the external ear and prevent auditory canal stenosis38. Han et al.39 reported a technique in which cartilage forms a strut for lateral extension between the cymba concha and the cavum concha so that cosmetic ear deformities are not seen. However, all patients underwent bilateral concha cartilage for rhinoplasty due to the limited amount available on the unilateral side10.
Concha cartilage can be harvested using either an anterior or posterior approach; of these, the anterior approach appears to experience fewer surgical complications. There have been reports of cosmetic ear deformities and hypertrophic scars; however, all studies were performed retrospectively. Furthermore, studies using an anterior approach have included a limited number of patients22,34. Consequently, complications may have been underreported. According to our review, the retroauricular approach is more popular. The most common complication was a postoperative hematoma, as increased exposure of the cartilage and skin of the posterior auricle involves many layers of tissue. As a result, blood vessels and the posterior auricular muscle can be injured and cause bleeding, which may be followed by hematoma if the bleeding is not well controlled. A retrospective approach appears safe; however, the gold standard of treatment cannot be concluded due to the retrospective manner of most studies. Therefore, a comparative or prospective cohort study is recommended to determine the best technique.
Concha cartilage seems to be the material of choice for septorhinoplasty among patients with cleft nasal deformity. The retroauricular approach is a simple and safe technique. In our series, 9.5% of patients experienced inadvertent postoperative conditions. The most common problem associated with harvesting is delayed wound healing, which can be quickly resolved using scrupulous surgical techniques during graft harvesting.
Acknowledgements
The authors thank (a) the staff and postgraduate students, including colleagues and co-workers, in the Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Mahidol University, for their aid and (b) Mr. Bryan Roderick Hamman for assistance with the English-language presentation of the manuscript.
Notes
Authors’ Contributions
S.T. participated in data collection, wrote the manuscript and performed data analysis. S.T., C.V., and K.B. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
This study was reviewed and approved by the institutional review board (IRB) of Faculty of Dentistry, Mahidol University (COA.No.MU-DT/PY-IRB 2022/051.0310), and the written informed consent was waived by the IRB due to the retrospective nature of the study.
References
1. Lim JS, Lee GT, Jung YS. 2012; Repair of bilateral cleft lip and nose by the Mulliken method: a case report. J Korean Assoc Oral Maxillofac Surg. 38:360–5. http://doi.org/10.5125/jkaoms.2012.38.6.360. DOI: 10.5125/jkaoms.2012.38.6.360.

2. Hsieh TY, Dedhia R, Del Toro D, Tollefson TT. 2017; Cleft septorhinoplasty: form and function. Facial Plast Surg Clin North Am. 25:223–38. https://doi.org/10.1016/j.fsc.2016.12.011. DOI: 10.1016/j.fsc.2016.12.011. PMID: 28340653.

3. Wong CH, Daniel RK, Lee ST. 2017; Asian cleft rhinoplasty: the open structural approach. Aesthet Surg J. 38:28–37. https://doi.org/10.1093/asj/sjx197. DOI: 10.1093/asj/sjx197. PMID: 29149246.

4. Cuzalina A, Tolomeo PG. 2021; Challenging rhinoplasty for the cleft lip and palate patient. Oral Maxillofac Surg Clin North Am. 33:143–59. https://doi.org/10.1016/j.coms.2020.09.012. DOI: 10.1016/j.coms.2020.09.012. PMID: 33246546.

5. Rohrich RJ, Benkler M, Avashia YJ, Savetsky IL. 2021; Secondary rhinoplasty for unilateral cleft nasal deformity. Plast Reconstr Surg. 148:133–43. https://doi.org/10.1097/prs.0000000000008124. DOI: 10.1097/PRS.0000000000008124. PMID: 34076624.

6. Hoang TA, Lee KC, Dung V, Chuang SK. 2022; Augmentation rhinoplasty in cleft lip nasal deformity using alloplastic material and autologous cartilage. J Craniofac Surg. 33:e883–6. https://doi.org/10.1097/scs.0000000000008848. DOI: 10.1097/SCS.0000000000008848. PMID: 35920855.

7. Ham J, Miller PJ. 2003; Expanded polytetrafluoroethylene implants in rhinoplasty: literature review, operative techniques, and outcome. Facial Plast Surg. 19:331–9. https://doi.org/10.1055/s-2004-815653. DOI: 10.1055/s-2004-815653. PMID: 14737702.

8. Keyhan SO, Ramezanzade S, Yazdi RG, Valipour MA, Fallahi HR, Shakiba M, et al. 2022; Prevalence of complications associated with polymer-based alloplastic materials in nasal dorsal augmentation: a systematic review and meta-analysis. Maxillofac Plast Reconstr Surg. 44:17. https://doi.org/10.1186/s40902-022-00344-8. DOI: 10.1186/s40902-022-00344-8. PMID: 35451637. PMCID: PMC9033909.

9. Mowlavi A, Pham S, Wilhelmi B, Masouem S, Guyuron B. 2010; Anatomical characteristics of the conchal cartilage with suggested clinical applications in rhinoplasty surgery. Aesthet Surg J. 30:522–6. https://doi.org/10.1177/1090820x10380862. DOI: 10.1177/1090820X10380862. PMID: 20829249.

10. Han Z, Wu B, Jiang B, Song P, Li G, Xu J. 2022; Effect of dichotomous conchal cartilage transplantation on correction of unilateral cleft lip nasal deformity. J Craniofac Surg. 33:592–6. https://doi.org/10.1097/scs.0000000000008060. DOI: 10.1097/SCS.0000000000008060. PMID: 34334746.

11. Lee M, Callahan S, Cochran CS. 2011; Auricular cartilage: harvest technique and versatility in rhinoplasty. Am J Otolaryngol. 32:547–52. https://doi.org/10.1016/j.amjoto.2010.11.008. DOI: 10.1016/j.amjoto.2010.11.008. PMID: 21316123.

12. Nolst Trenité GJ, Paping RH, Trenning AH. 1997; Rhinoplasty in the cleft lip patient. Cleft Palate Craniofac J. 34:63–8. https://doi.org/10.1597/1545-1569_1997_034_0063_ritclp_2.3.co_2. DOI: 10.1597/1545-1569_1997_034_0063_ritclp_2.3.co_2. PMID: 9003914.

13. Boccieri A, Marano A. 2007; The conchal cartilage graft in nasal reconstruction. J Plast Reconstr Aesthet Surg. 60:188–94. https://doi.org/10.1016/j.bjps.2006.02.005. DOI: 10.1016/j.bjps.2006.02.005. PMID: 17223517.

14. Wee JH, Park MH, Oh S, Jin HR. 2015; Complications associated with autologous rib cartilage use in rhinoplasty: a meta-analysis. JAMA Facial Plast Surg. 17:49–55. https://doi.org/10.1001/jamafacial.2014.914. DOI: 10.1001/jamafacial.2014.914. PMID: 25429595.

15. Kozlov MI. 1998; [Chondroperichondritis of costal cartilages as a complication of surgical approaches to the thorax without direct injury of the cartilage]. Vestn Khir Im I I Grek. 157:48–50. Russian. PMID: 9691381.
16. Nanda V, Sharma RK, Tuli P, Makkar S. 2005; Costal cartilage necrosis--an unusual complication of electric injury. Burns. 31:665–7. https://doi.org/10.1016/j.burns.2005.01.007. DOI: 10.1016/j.burns.2005.01.007. PMID: 15955635.

17. Rhomberg W, Schuster A. 2014; Premature calcifications of costal cartilages: a new perspective. Radiol Res Pract. 2014:523405. https://doi.org/10.1155/2014/523405. DOI: 10.1155/2014/523405. PMID: 25587444. PMCID: PMC4284933.

18. Lu TC, Yao CF, Lin S, Chang CS, Chen PK. 2017; Primary septal cartilage graft for the unilateral cleft rhinoplasty. Plast Reconstr Surg. 139:1177–86. https://doi.org/10.1097/prs.0000000000003297. DOI: 10.1097/PRS.0000000000003297. PMID: 28098711.

19. Zhang L, Bai X, Li Z, Liu Q, Yang M, Wang X, et al. 2018; Improvement of aesthetic and nasal airway in patients with cleft lip nasal deformities: rhinoplasty with septal cartilage graft and septoplasty. Cleft Palate Craniofac J. 55:554–61. https://doi.org/10.1177/1055665617746260. DOI: 10.1177/1055665617746260. PMID: 29554442.

20. Lee JS, Lee DC, Ha DH, Kim SW, Cho DW. 2016; Redefining the septal L-strut to prevent collapse. PLoS One. 11:e0153056. https://doi.org/10.1371/journal.pone.0153056. DOI: 10.1371/journal.pone.0153056. PMID: 27073993. PMCID: PMC4830535.

21. Hantawornchaikit T, Arayasantiparb R, Kumar K, Boonsiriseth K. 2022; Three-dimensional analysis of definitive secondary unilateral cleft rhinoplasty using cartilage graft. Cleft Palate Craniofac J. 59:1072–8. https://doi.org/10.1177/10556656211034099. DOI: 10.1177/10556656211034099. PMID: 34402317.

22. Wright ST, Calhoun KH, Decherd M, Quinn FB. 2007; Conchal cartilage harvest: donor site morbidities, patient satisfaction, and cosmetic outcomes. Arch Facial Plast Surg. 9:298–9. https://doi.org/10.1001/archfaci.9.4.298. DOI: 10.1001/archfaci.9.4.298. PMID: 17638769.

23. Kim JY, Yang HJ, Jeong JW. 2017; A new technique for conchal cartilage harvest. Arch Plast Surg. 44:166–9. https://doi.org/10.5999/aps.2017.44.2.166. DOI: 10.5999/aps.2017.44.2.166. PMID: 28352607. PMCID: PMC5366525.

24. Jovanovic S, Berghaus A. 1991; Autogenous auricular concha cartilage transplant in corrective rhinoplasty. Practical hints and critical remarks. Rhinology. 29:273–9. PMID: 1780628.
25. Nemoto T, Matsui Y, Shirota T, Irie T, Tachikawa T. 2008; Two cases of scar formation in donor site for conchal cartilage grafting. J Jpn Stomatol Soc. 57:278–82. https://doi.org/10.11277/stomatology1952.57.278.

26. Du H, Zhang D, Zong X, Song G, Zhao J, Yang C, et al. 2021; A simple dressing fixation method following harvest of the ear cartilage graft. J Craniofac Surg. 32:e240–2. https://doi.org/10.1097/scs.0000000000006965. DOI: 10.1097/SCS.0000000000006965. PMID: 32890175.

27. Muenker R. 1984; The bilateral conchal cartilage graft: a new technique in augmentation rhinoplasty. Aesthetic Plast Surg. 8:37–42. https://doi.org/10.1007/bf01572783. DOI: 10.1007/BF01572783. PMID: 6731159.

28. Mudry A, Pirsig W. 2009; Auricular hematoma and cauliflower deformation of the ear: from art to medicine. Otol Neurotol. 30:116–20. https://doi.org/10.1097/mao.0b013e318188e905. DOI: 10.1097/MAO.0b013e318188e905. PMID: 18800018.

29. Mohan V, Bhavani S, Subramanian SK, Maiti A. 2017; Calcified cauliflower ear in relapsing polychondritis. BMJ Case Rep. 2017:bcr2017219424. https://doi.org/10.1136/bcr-2017-219424. DOI: 10.1136/bcr-2017-219424. PMID: 28432170. PMCID: PMC5534701.

30. Zilinsky I, Erdmann D, Weissman O, Hammer N, Sora MC, Schenck TL, et al. 2017; Reevaluation of the arterial blood supply of the auricle. J Anat. 230:315–24. https://doi.org/10.1111/joa.12550. DOI: 10.1111/joa.12550. PMID: 27726131. PMCID: PMC5244454.

31. Lan MY, Park JP, Jang YJ. 2017; Donor site morbidities resulting from conchal cartilage harvesting in rhinoplasty. J Laryngol Otol. 131:529–33. https://doi.org/10.1017/s0022215117000639. DOI: 10.1017/S0022215117000639. PMID: 28316288.

32. Ro HS, Roh SG, Shin JY, Lee NH, Yang KM. 2017; Iatrogenic through-and-through conchal defect secondary to auricular cartilage graft. Aesthetic Plast Surg. 41:56–9. https://doi.org/10.1007/s00266-016-0764-0. DOI: 10.1007/s00266-016-0764-0. PMID: 28032171.

33. Kim SW, Park CW, Kim JT, Kim YH. 2012; Merocel semicompressive dressing to prevent donor-site hematoma on the conchal cartilage graft. J Craniofac Surg. 23:57–60. https://doi.org/10.1097/scs.0b013e318240c908. DOI: 10.1097/SCS.0b013e318240c908. PMID: 22337374.

34. Grobbelaar AO, Matti BA, Nicolle FV. 1997; Donor site morbidity post-conchal cartilage grafting. Aesthetic Plast Surg. 21:90–2. https://doi.org/10.1007/s002669900090. DOI: 10.1007/s002669900090. PMID: 9143422.

35. Elazhary EA, Abd Al-Salam FM, Abd El-Hafiz HS, Maghraby HK. 2022; Updates on keloid scar pathogenesis, assessment and treatment modalities. J Recent Adv Med. 3:75–86. DOI: 10.21608/jram.2021.82892.1123.

36. Butt MF, Albusoda A, Farmer AD, Aziz Q. 2020; The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat. 236:588–611. https://doi.org/10.1111/joa.13122. DOI: 10.1111/joa.13122. PMID: 31742681. PMCID: PMC7083568.

37. Deleyiannis FW. 2010; Reconstruction of the concha to restore hearing after cartilage harvest for rhinoplasty. Arch Otolaryngol Head Neck Surg. 136:304–5. https://doi.org/10.1001/archoto.2010.23. DOI: 10.1001/archoto.2010.23. PMID: 20231653.

38. Adams WM. 1955; Construction of upper half of auricle utilizing composite concha cartilage graft with perichondrium attached on both sides. Plast Reconstr Surg (1946). 16:88–96. https://doi.org/10.1097/00006534-195508000-00002. DOI: 10.1097/00006534-195508000-00002. PMID: 13254361.

39. Han K, Kim J, Son D, Park B. 2008; How to harvest the maximal amount of conchal cartilage grafts. J Plast Reconstr Aesthet Surg. 61:1465–71. https://doi.org/10.1016/j.bjps.2007.09.038. DOI: 10.1016/j.bjps.2007.09.038. PMID: 17996506.

Fig. 1
Surgical procedure of concha cartilage harvesting by using retroauricular approach. A. An incision was made in a curved fashion. B. Subperichondrial dissection was done at the posterior surface of the concha cartilage. C, D. Short gauge 30 hypodermic needles were used to set boundaries of the harvesting area. E. Hemostasis was done. F. Bolster gauze dressing. G. The subcuticular suture was performed with an inserted Penrose drain. H. The harvested concha cartilage.
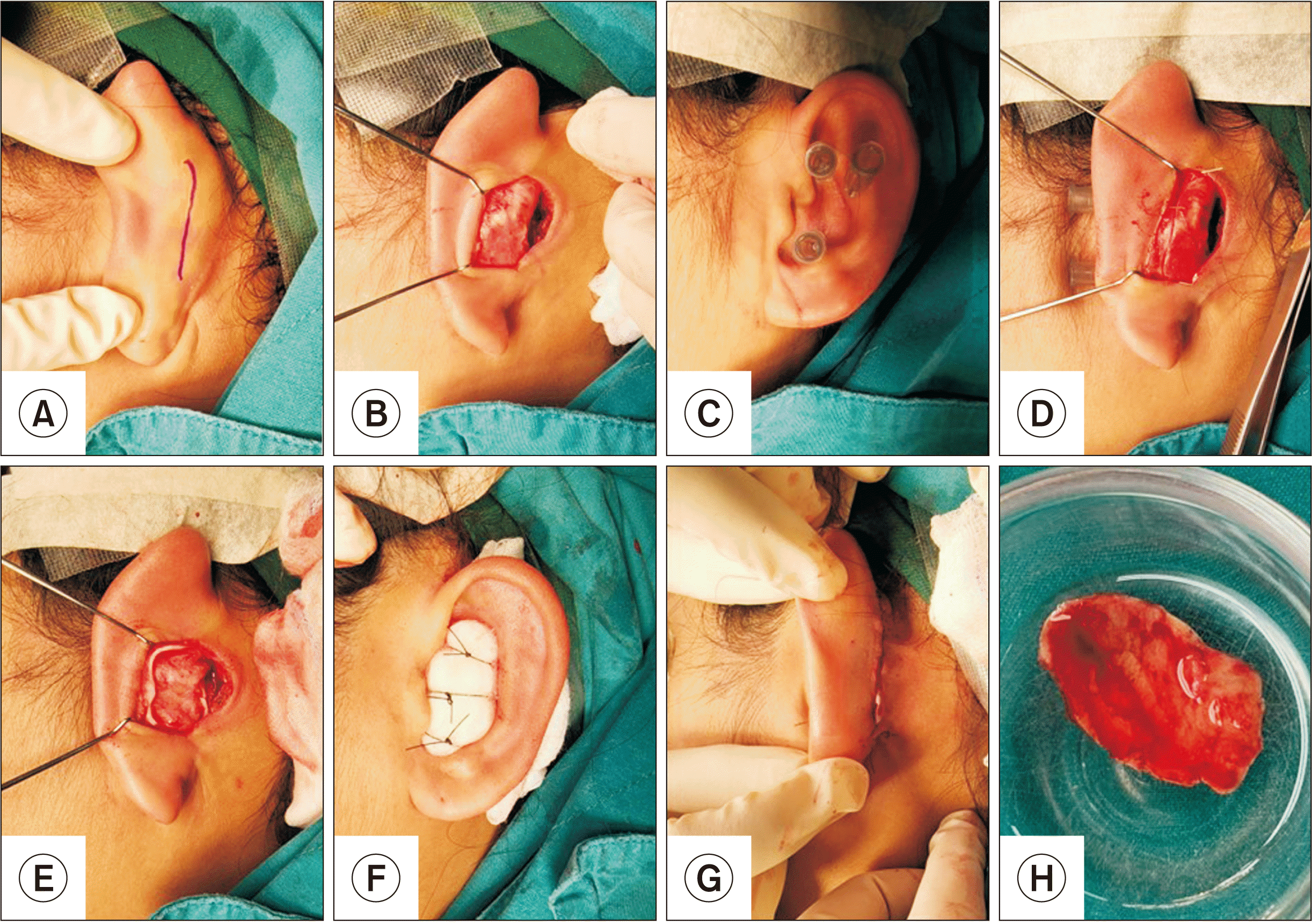
Fig. 2
The patient with dehiscence wound. A. Dehiscence wound size 5 mm. B. The wound was resutured in a simple interrupted manner. C. Two weeks after resuturing, the wound was healed.
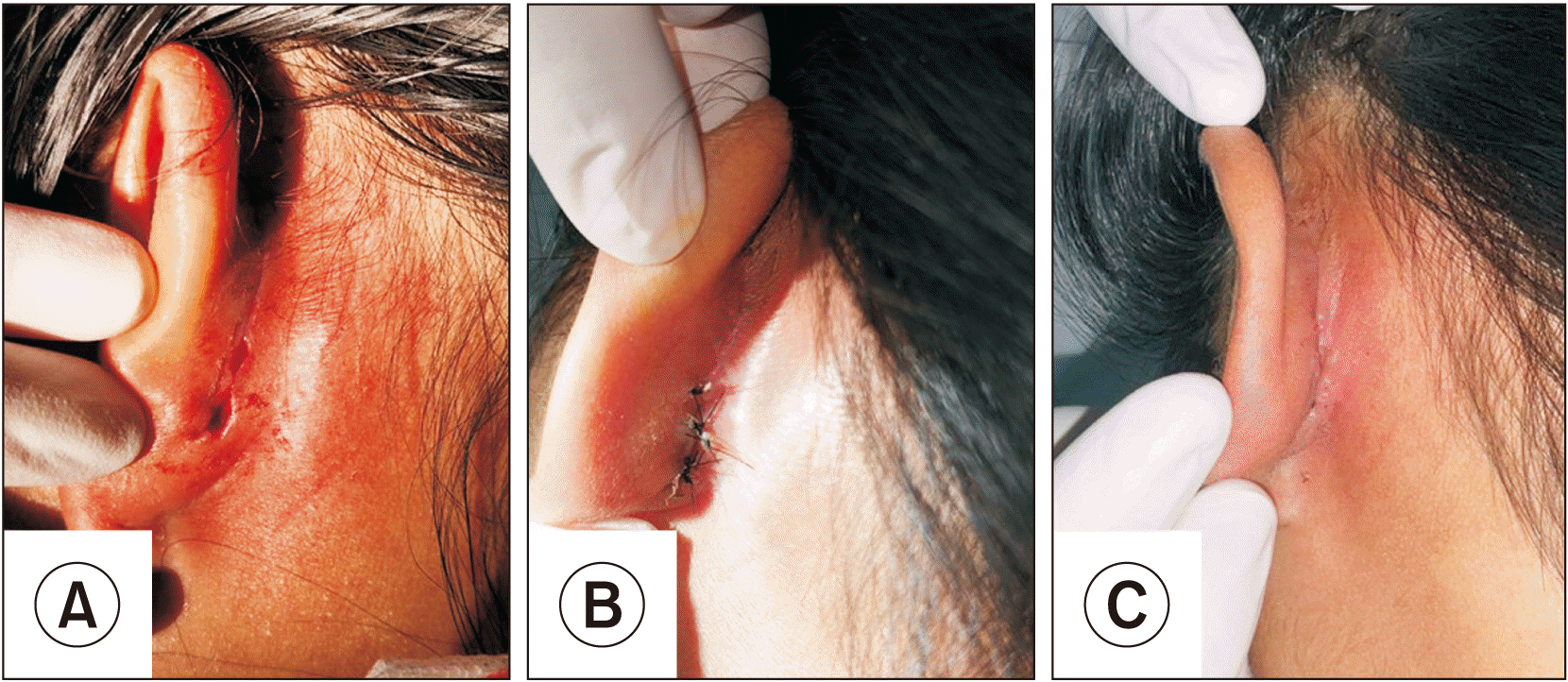
Table 1
Demographic data of patients
Table 2
Complications associated with concha cartilage harvesting




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download