INTRODUCTION
Sudden cardiac arrest is defined as the sudden cessation of cardiac activity with no sign of normal respiration and circulation, which is a medical emergency that progresses to sudden cardiac death (SCD) if not managed immediately.
1) Ischemic heart disease is the most common and serious burden predisposing patients to SCD, followed by cardiomyopathy.
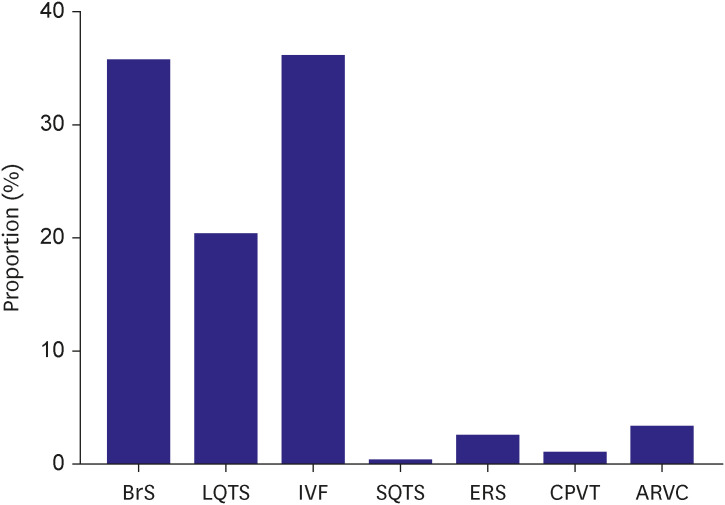
2)3) Inherited arrhythmia (IA) is a primary electric disorder caused by genetic alterations in cardiac ion channel proteins and comprises various inherited diseases such as Brugada syndrome (BrS), long QT syndrome (LQTS), and idiopathic ventricular fibrillation (IVF). IA is rare cardiac cause of SCD, and is more prevalent in the Asian population.
4)5)6)
There has been difficulty in the clinical diagnosis of IA in patients with insufficient electrical evidence. Advances in genetic testing methods have led to the identification of unrevealed genotypes, and have shown potential as diagnostic and prognostic tools for inherited cardiovascular disease. In other words, genetic testing using a multi-gene panel might provide a molecular diagnosis of underdiagnosed SCD, which is promising in the diagnosis of IA.
Although the significance of IA is more accentuated in Asian populations, little is known about its clinical characteristics and genetic background in Asian populations. To date, most genetic studies in Asian IA have been based on Japanese cohorts, and a multicenter registry with a large volume of IA probands is lacking. Therefore, based on a multicenter Korean IA cohort, we aimed to investigate the clinical characteristics of Korean IA probands and further analyze the genetic findings via next-generation sequencing (NGS).
METHODS
Ethical statement
This study was approved by the institutional review board of each hospital (IRB No. 2014AN0104), and informed consent was obtained from all patients.
Study population
This study was based on a multicenter cohort of the Korean IA Registry, which included 401 probands and family members in 14 tertiary centers from February 2014 to November 2017 (
Supplementary Table 1).
The inclusion criteria of IA probands conformed to the Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society expert consensus statement on the diagnosis during study period, and the inclusion was confirmed by an electronic case report form to standardize patient inclusion from different centers.
7) In our cohort, IA included seven diseases: BrS, LQTS, short QT syndrome (SQTS), catecholaminergic polymorphic ventricular tachycardia (CPVT), early repolarization syndrome, IVF, and arrhythmogenic right ventricular cardiomyopathy (ARVC). Probands were excluded if they had 1) significant coronary artery disease, 2) systemic disease such as malignancy with a life expectancy of less than one year, and 3) cardiomyopathy except ARVC. Family members of probands (siblings, parents, or children) were enrolled if they had not been diagnosed with IA previously and agreed for enrollment and data collection for the study. Eight participants were excluded because of missing data, and the registry comprised 265 IA probands and 128 family members.
Data collections
Clinical data were collected using an electronic case report form. The data consisted of baseline characteristics, including age at the time of diagnosis, sex, family history of IA and SCD, body mass index, symptoms or signs at diagnosis, history of ventricular tachycardia or ventricular fibrillation, and underlying comorbidities. Further clinical data including electrocardiographic and echocardiographic findings, provocation test results (epinephrine and sodium channel blocker), current medications, and implantable cardioverter-defibrillator (ICD) implantations were also collected. Genetic testing was recommended for all patients. Those without informed consent for genetic analysis and its expenses were excluded for further genetic testing.
Genetic testing using next-generation sequencing panel
Among the 265 probands, genetic testing by NGS was performed on 216 probands. Genomic DNA was extracted from peripheral blood leukocytes using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). A Celemics Customized Target Enrichment Kit (Celemics, Seoul, Korea) was used for library preparation. Sequencing was performed using the hybrid capture method by generating 2×150 base pairs paired-end reads on the Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA). Sequencing was performed using 174 multigene panels designed by Illumina Design Studio (Illumina Inc.) to investigate the 3,130 target lesions known to cause cardiovascular disease according to the Human Gene Mutation Database (HGMD,
http://www.hgmd.cf.ac.uk) (
Supplementary Table 2).
Alignment of sequence reads, indexing of the reference genome, hg19 (GRCh37), and variant calling were conducted with a pipeline based on GATK Best Practice (alignment was performed with the BWA-mem [version 0.7.12], duplicated reads were marked with Picard [version 1.96,
http://picard.sourceforge.net] and local alignment, base quality recalibration, variant calling was performed with the Genome Analysis Tool kit [GATK, version 3.2-2], and annotation was performed with VEP [Variant Effect Predictor], dbNSFP v3.02.).
The data were analyzed in the following three steps. The primary analysis was sequence generation, which converts sequenced raw data into a text-based format (FASTQ) to store both biological sequences and the corresponding quality scores. Secondary analysis involved sequence processing with variant calling, which selects a different sequence (variant) compared with the reference sequence. Tertiary analysis included validation and clinical interpretation by database and algorithms (evidence-based, frequent-based, functional, and predictive).
8)
Bioinformatics
Filtering of false positive variants was performed as follows: the region coding the actual protein from the flanking region was identified; only loss of function variants (frameshift, nonsense, consensus splice site) were selected in order to distinguish mutations causing the actual variant; variants with allele frequency of more than 0.3 were included; minor allele frequency of more than 5% in population frequency database was excluded; compound heterozygous variants in autosomal recessive inheritance genes were included; and the disease-causing mutation reported only in HGMD was included.
The following criteria were used for validation of analysis accuracy: read depth was at least 10X or higher for increased sensitivity, variants with 10X coverage of 90% or more were included, the number of calling variants was at least 100 (per batch).
Multiple databases were used to analyze the variants. The variant allele frequency and minor allele frequency were determined according to the following population databases:1000 Genome Project (
http://www.internationalgenome.org/), Exome Aggregation Consortium (ExAC,
http://exac.broadinstitute.org), single nucleotide polymorphism database (dbSNP,
https://www.ncbi.nlm.nih.gov/SNP/), and Korean reference genome database (KRGDB,
http://coda.nih.go.kr/coda/KRGDB/index.jsp). The association with IA was determined using disease databases, including HGMD, Clinvar (
https://www.ncbi.nlm.nih.gov/clinvar/), and OMIM (
http://www.omim.org/). Different in-silico analysis tools including SIFT (
https://sift.bii.a-star.edu.sg/), Polyphen2 (HDIV/HVAR) (
http://genetics.bwh.harvard.edu/pph2/), likelihood-ratio test, Mutation taster/assessor (
http://www.mutationtaster.org/,
http://mutationassessor.org/), FATHMN (
http://fathmm.biocompute.org.uk), PROVEAN (
http://provean.jcvi.org) were harnessed to determine the probabilities of variant pathogenicity.
Variant classifications
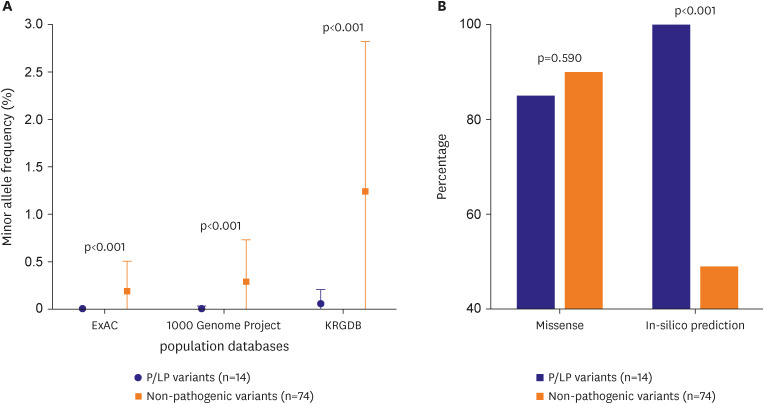
Variants were classified into five categories (pathogenic, likely pathogenic, variant of unknown significance, likely benign, or benign) based on the Standard and Guidelines for the Interpretation of Sequence Variant of American College of Medical Genetics and Genomics and the Association for Molecular Pathology.
9) Genotypes were defined as positive if the following three analytic criteria were met: 1) variant is in disease-associated gene according to American College of Medical Genetics and Genomics classification,
10)11) 2) variant allele frequency is more than 0.3, and 3) minor allele frequency is less than 5%. Genetic yield was defined as ratio of probands detected with positive genotypes, and was further specified as ‘yield of pathogenic or likely pathogenic (P/LP) variants.’ Variant analysis and interpretation were curated by clinical cardiologists and genetic bioinformatics specialists.
Statistical analysis
Categorical variables were described as numbers and percentages, and continuous variables were presented as means and standard deviations. For comparison of categorical and continuous variables, Student’s t-test, Mann-Whitney U test, χ2 test, and Fisher’s exact test were used as indicated. One-way analysis of variance (ANOVA) was used to compare the three variables. All tests were two-tailed, and a p-value of ≤0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 26; IBM Corp., Armonk, NY, USA).
DISCUSSION
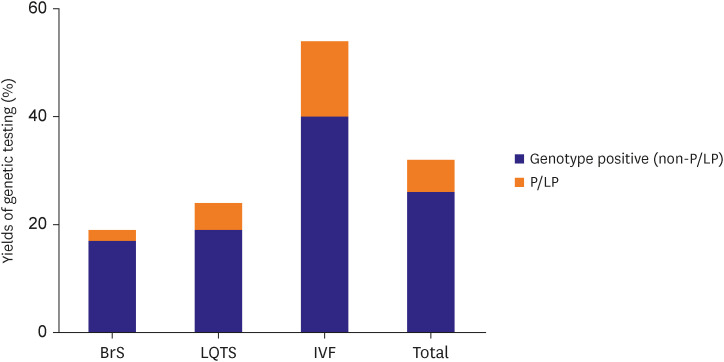
Based on the multicenter IA registry of Korea, this study investigated clinical characteristics and further assessed the genetic background of IA probands using an NGS panel. Major findings of this study can be summarized as follows: First, the most common disease entity was IVF, followed by BrS, and LQTS. Second, certain differences in clinical characteristics were demonstrated among the three disease entities, including sex differences and lethal arrhythmic events. Third, there was significant difference of left ventricular ejection fraction between positive and negative genotype probands (54.7±11.3 vs. 59.3±9.2%, p=0.005). Lastly, the genetic testing yield was relatively low for BrS and LQTS; however, IVF revealed a higher genetic testing yield, which was supported by a substantial proportion of cardiomyopathy-related variants.
IVF, BrS, and LQTS are the three most common disease entities, which accounted for more than 90% of whole Korean IA population. The disease was usually diagnosed in the fourth decade, with a slightly earlier onset of LQTS. In addition, BrS and IVF were predominant in males and LQTS in females. Compared to BrS or LQTS, the IVF subgroup encountered significantly more lethal arrhythmic events, which led to a higher incidence of ICD implantation. This may be explained by the current diagnostic criteria for each disease, where electrocardiographic abnormality is the major diagnostic criterion for BrS and LQTS, whereas an event of unexplained cardiac arrest is required to diagnose IVF.
Clinical characteristics of probands were compared depending on the presence of genotypes; there was no difference between genotype positive and genotype negative probands except for those with left ventricular ejection fraction. The exact mechanism for the lower left ventricular ejection fraction in the positive genotype remains unclear, but it might be partly explained by concept of concealed cardiomyopathy in IVF, since IVF probands resulted highest genetic yield.
12)13) In other words, clinically overt cardiomyopathy might be preceded by a preclinical phase, and sudden cardiac arrest might be the first cardiac manifestation in this population. Nevertheless, this finding should be interpreted with caution. The numerical differences of left ventricular ejection fraction between two groups were observed by simple comparison, and do not address direct cause-and-effect relationship with genetic result. In addition, these differences were not substantial (54.7±11.3 vs. 59.3±9.2%). Although lower left ventricular ejection fraction in positive genotypes may not alter treatment strategy in clinical practice, it may support consistent monitoring of structural change as well as possibility of progression to clinical cardiomyopathy in genotype positive probands. Advanced research specified in each disease entity is needed to fully interpret this finding.
In our study, genetic yield for via the NGS panel was found to be lower than that reported in previous studies.
14)15) Although
SCN5A was the most common genotype detected in BrS probands, the genetic yield of
SCN5A was 10.9% (9 out of 82 probands), which was lower than that reported previously reported (approximately 20%). Non-
SCN5A variants (
CACNA1C,
SCN1B,
HCN4,
CACNB2,
PKP2,
and SCN3B) were also prevalent and mostly rare. However, only two
SCN5A variants were identified as P/LP variants, which is consistent with previous evidence that
SCN5A is the only definitive molecular marker for BrS.
16)
In LQTS, more than 75% probands are reported to have three major genes (
KCNQ1, KCNH2, SCN5A) in western population and in a Japanese cohort.
17)18) In previous studies of Korean LQTS probands, LQTS genes were detected in 50–70% of probands.
19)20) The NGS panel utilized in this study included all LQTS-related genes that have definite or strong evidence for causality of LQTS except
CALM3.
21) In addition, our study utilized a larger genetic panel, but genetic testing including multiple genes did not improve the diagnostic yield in the LQTS subgroup. There may be several reasons for relatively low yield in LQTS. First, the NGS panel used in our study was developed based on a western genetic background, which may not be fully reflect genetic background of Asian IA probands. Second, thorough segregation study of familial genetic screening was not done in every proband, which have weakened the pathogenicity classified by American College of Medical Genetics and Genomics. Third, there may be selection bias that led to overdiagnosis of LQTS. LQTS is likely to be overdiagnosed due to clinical symptom such as vasovagal syncope, and transient QT interval prolongation during stress condition.
22) In our data, LQTS revealed higher incidence of syncope as well as lower incidence of aborted SCD, which implies possibility of overdiagnosis in LQTS.
In contrast to BrS and LQTS, the genetic yield of IVF was substantial in our study. More than half of the patients were detected with certain genotype, and 13.5% had P/LP variants. Our findings were consistent with previous studies in Caucasian IVF population that revealed 10–18% of cardiomyopathy-related P/LP variants.
23)24) A high incidence of IVF indicates a paucity of clinical phenotypes to identify the cause of ventricular fibrillation or SCD at the initial examination. In addition, the higher genetic detection rate in IVF is mainly due to the cardiomyopathy-related P/LP variant found in this specific group. ARVC exhibits a preclinical phase that does not express evident structural abnormality.
25) Similarly, hypertrophic cardiomyopathy may not be diagnosed at initial phase, if left ventricular wall thickness is equivocal or in borderline range.
26) Our finding implies the possibility of concealed cardiomyopathy in this population, which suggests serial imaging follow-up, such as echocardiography, in IVF probands to undercover disease progression to overt cardiomyopathy.
Therefore, regarding the genetic yield of NGS panels in Korean IA probands, genetic testing using a large-volume gene panel did not provide better yield in BrS and LQTS. On the other hand, in IVF, multiple genetic sequencing, such as NGS or whole-exome sequencing, may enable molecular diagnosis of concealed phenotypes, which can change future management strategies, such as further imaging studies or additional familial screening.
This study was based on the first multicenter registry of Korean IA probands and utilizes a large genetic testing panel including 174 genetic variants of cardiovascular disease. It provided comprehensive information on the clinical and genetic characteristics of the three most common disease entities of IA. More studies are needed in Korean IA probands in order to improve our understanding of the genetic mechanism, and further establish genotype-phenotype correlation in IVF to identify concealed cardiomyopathy.
This study had several limitations. First, the epidemiologic distribution of this registry may not represent the actual prevalence of each disease in Korea. Although IA probands were enrolled regardless of symptoms, the registry resulted high proportion of sudden cardiac arrest survivor (80.8% in total probands), which might lead to selection bias that increase proportion of IVF. It may have resulted from the clinical need of genetic testing in sudden cardiac arrest of unknown origin (or IVF) to identify genetic susceptibility to specific disease. Second, it focused on more common diseases of IA and provides limited information on other rare diseases, such as early repolarization syndrome, CPVT, and SQTS. Considering its prevalence, further studies with larger numbers of sudden cardiac arrest survivors are needed to encompass the rare diseases of IA. Third, clinical information may be limited due to the retrospective nature of the data. Most of the clinical information was obtained at the time of sudden cardiac arrest or admission, which may reflect worse cardiac function (left ventricular ejection fraction) or cardiac conduction property. In this regard, further collection of follow-up data might supplement this shortcoming. Lastly, although many cardiomyopathy-related genotypes were found in IVF, IVF-related rare variants such as DPP6 or CALM1 were not identified in this population. However, this study provides important insights into genetic backgrounds of IA in Korea although further study for genetic data including rare IAs.
BrS IVF, and LQTS were three major disease entities in Korean IA probands, which differed not only in their clinical characteristics but also in the arrhythmic event rate that led to ICD implantation. Genotype positive probands showed lower left ventricular ejection fraction compared to genotype negative probands. The genetic yield of BrS and LQTS was lower than previous studies, even with the use of the large-volume NGS panel. In IVF probands, genetic screening for cardiovascular disease-associated genes may be promising to unveil hidden phenotype such as concealed cardiomyopathy.







 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download