Abstract
Background
Recent studies have reported that costoclavicular blocks (CCBs) can consistently block almost all branches of the brachial plexus while sparing the phrenic nerve and provide effective analgesia after shoulder surgery. We aimed to compare the efficacy of the CCB with that of the interscalene block (ISB) as the sole blocking technique for shoulder surgery.
Methods
A total of 212 patients undergoing elective arthroscopic shoulder surgery were randomized to receive an ISB or CCB based on a non-inferiority design. All patients received titration sedation with propofol under monitored anesthesia during surgery. The primary outcomes were the proportion of patients with complete motor blockade of the suprascapular nerve (SSN) and incidence of hemidiaphragmatic paralysis (HDP). The secondary outcomes included block-related variables, complications, and postoperative pain scores.
Results
The proportion of patients with complete motor blockade of the SSN at 20 min between the CCB and ISB groups (53% vs. 66%) exceeded the predefined non-inferiority margin of −5%, but was comparable at 30 min (87% vs. 91%). The CCB resulted in a significantly lower incidence of HDP (7.55% vs. 92.45%), Horner’s syndrome (0% vs. 18.87%), and dyspnea (0% vs. 10.38%) than the ISB. None of the patients experienced failed blocks or required conversion to general anesthesia. Pain scores were comparable between the groups.
Multiple modes of anesthesia and analgesia, such as general anesthesia (GA), interscalene blocks (ISBs), various brachial plexus block approaches, and selective nerve blocks in combination with GA, have been effective in shoulder surgery [1]. Identifying the optimal regional anesthesia for shoulder surgery is essential for improving postoperative recovery in a day-surgery setting [2]. Inadequate treatment of postoperative pain and side effects associated with GA, such as somnolence and postoperative nausea and vomiting (PONV), result in delayed discharge and readmission [3,4]. ISBs are a standardized and reliable technique for postoperative analgesia after arthroscopic shoulder surgery and are also sometimes used as the sole means of anesthesia [4–6]. However, ISBs are associated with a relatively high risk of hemidiaphragmatic paralysis (HDP) and nerve injury [7,8]. Although several brachial plexus blocks distal to the interscalene approach have been proposed as diaphragm-sparing techniques [9], to date, only the costoclavicular block (CCB) has been found to provide postoperative analgesia equivalent to that of the ISB along with the lowest incidence of HDP [10].
The CCB, which was first described by Karmakar et al. [11] in 2015, targets the three cords that originate from the division and fusion of the brachial plexus in the costoclavicular space. Recently, Koyyalamudi et al. [12] reported in their cadaveric study that a single injection of 0.1% methylene blue (20 ml) in the costoclavicular space could consistently spread cephalad to all trunks and divisions in the supraclavicular space while sparing the phrenic nerve. Although only five specimens were included in that study, the findings also anatomically confirm the assumption of a “retrograde channel” between the costoclavicular and supraclavicular spaces [13]. The potential ability of the CCB providing surgical anesthesia for arthroscopic shoulder surgery may be recognized by the unexpected diffusion of local anesthetics (LA). This led us to speculate that if we could anesthetize almost all myotome and osteotome innervations of the shoulder joint, we could increase the acceptability of the CCB for intraoperative surgical anesthesia [14,15]. To date, clinical studies evaluating the efficacy and safety of CCBs as the sole anesthetic technique for proximal upper limb surgery have been limited [16,17].
The suprascapular nerve (SSN) and axillary, subscapular, and lateral pectoral nerves innervate the vast majority of the sensorimotor system of the shoulder joint and its adjacent structures [15]. The SSN arises from the superior trunk in the proximal trajectory of the brachial plexus, whereas the remaining three nerves originate from the cord level of the brachial plexus (in the costoclavicular space). Because of the block characteristics of the CCB, identifying whether LA can spread to the SSN in clinical practice and validating the bold assumption that it can be used as the sole means of anesthesia for arthroscopic shoulder surgery is therefore urgent. In this prospective randomized trial, we compared ultrasound-guided single-injection ISBs to CCBs, with additional supraclavicular nerve blocks provided per the surgical procedure. We reasoned that the CCB could potentially obtain a sensory-motor blockade similar to that of the ISB as it benefited from the consistent cephalad spread of the LA. Consequently, we designed this study as a non-inferiority trial and hypothesized that for arthroscopic shoulder surgery, the CCB would result in block characteristics of the SSN and success rates of sensory and/or motor blockades of the important nerves innervating the shoulder joint over time similar to those of the conventional ISB, while reducing the risk of HDP.
This trial was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (No. JY-2020-213) and prospectively registered in the China Clinical Trial Registry on December 11, 2020 (No. ChiCTR2000040841). This study was also conducted in accordance with the ethical principles of the Helsinki Declaration 2013. After providing written informed consent, 212 patients were enrolled and scheduled for elective unilateral arthroscopic shoulder surgery between December 14, 2020, and December 30, 2021. The patients were aged between 18 and 75 years and had American Society of Anesthesiologists (ASA) physical status scores of I, II, or III. The exclusion criteria were as follows: refusal to participate, pre-existing moderate-severe pulmonary disease (obstructive or restrictive), pre-existing neuropathy of the operative limb, infraspinatus muscle injury, coagulopathy, hepatic failure (Child-Turcotte-Pugh score > 9) or renal failure (creatinine ≥ 2 mg/dl or peritoneal dialysis or hemodialysis), infection at the puncture site, hypersensitivity or allergy to LA, body mass index > 35 kg/m2, pregnancy, inability to communicate or cooperate, anticipated difficult airway, or chronic pain condition.
The study was conducted at our day surgery center using a randomized, controlled, parallel-group design based on the 2010 Consolidated Standards of Reporting Trials (CONSORT) guidelines. After enrollment, the participants were randomly allocated to either the control group (ISB group, n = 106) or experimental group (CCB group, n = 106) at a 1 : 1 ratio by computer-generated simple randomization. On the day of surgery, a previously prepared and sealed opaque envelope containing the random group assignment was opened by a research assistant who was not involved in this study. The group allocation was then conveyed to the block practitioner before block performance. To eliminate performance bias, all blocks were performed by experienced regional anesthesiologists. All anesthesiologists in charge of intraoperative anesthesia management, outcome assessors, patients, and follow-up personnel were blinded to group allocation.
None of the patients received any premedication. After entering the dedicated block room, the patients were connected to standard ASA monitors, which included noninvasive cuff blood pressure, pulse oxygen saturation, and 5-lead electrocardiography measurements. An 18-gauge or 20-gauge intravenous (IV) cannula for fluid infusion was placed in the contralateral forearm, and premedication (IV midazolam 2 mg and IV fentanyl 0.5 µg/kg) was administered for anxiolytic effect before the block procedure. After sterilizing and infiltrating the skin with 2% lidocaine (2–3 ml), the nerve block was performed according to group allocation using a portable ultrasound machine (SonoSite M-turbo, SonoSite, Inc., USA) with a 6–15 MHz high-frequency linear array transducer and a 22-gauge, 80-mm insulated stimulating needle (B. Braun Melsungen AG, Germany).
The ultrasound-guided ISB was performed according to the technique described by Kang et al. [18] after a satisfactory image of the C5 and C6 nerve roots (hypoechoic ellipse or circle) in the short axis in the interscalene region was obtained. The needle was advanced using the in-plane technique to the ultrasound beam in a lateral-to-medial direction through the middle scalene muscle under real-time ultrasound imaging. The targeted needle tip was positioned immediately lateral to the brachial plexus sheath and between the C5 and C6 nerve roots, where 20 ml of 0.5% ropivacaine was injected incrementally.
For the CCB, the needling technique was performed according to the method described by Aliste et al. [10]. The patients were placed in the supine position with the surgical limb at 90° of abduction. The ultrasound transducer was initially placed directly on top of the middle third of the clavicle, and then the probe was moved on the inferior border of the clavicle and towards the medial infraclavicular fossa in the medial-to-lateral direction. Once the three cords of the brachial plexus and axillary artery were identified at the costoclavicular space along the short axis, the operator accurately oriented the needle tip to the center of the three cords using the in-plane technique and advanced in the lateral-to-medial direction. After negative aspiration, 20 ml of 0.5% ropivacaine was injected in divided doses. Finally, all the patients in both groups received an ultrasound-guided ipsilateral supraclavicular nerve block with 3 ml of 0.5% ropivacaine immediately, according to the method described by Maybin et al. [19].
After undergoing a post-block assessment, all patients were transferred to the operating room where they were placed in the lateral decubitus position (operative side up) and underwent monitored anesthesia care (MAC) with Narcotrend monitoring. In the event that the block failed, rescue measures, including supplemental narcotics (e.g., 25 μg IV fentanyl boluses), rescue blocks, infiltration of LA, or conversion to GA, were performed according to the treating anesthesiologists’ discretion. Oxygen supplementation (pure oxygen) was administered through a face mask at a gas flow rate of 4 L/min. A single bolus dose of 0.5–1.5 mg/kg IV propofol over 10 min was administered to achieve a targeted Ramsay sedation scale (RSS) score of 3 (response to command). A supplementary bolus dose of 0.2 mg/kg IV propofol was administrated intermittently until the RSS reached level 3, after which a continuous IV infusion of 1.0–2.5 mg/kg/h propofol was administered to achieve a moderate level of sedation, as assessed by the Narcotrend index (C0–C2) during surgery. Titrated sedation with propofol and vasopressors (as needed) was used to maintain the heart rate and mean arterial blood pressure within 20% of baseline measurements during surgery.
After surgery, propofol was discontinued and the patients were transferred to the post-anesthesia care unit (PACU). The severity of postoperative pain was assessed by the nurses using a 10-point numerical rating scale (NRS: 0 = no pain, 10 = worst imaginable pain) at 15-min intervals. Patients with an NRS score > 4 received 2 mg IV morphine every 10–15 min until they were comfortable (NRS < 4). All patients received 200 mg oral celecoxib every 12 h supplemented with 2 mg IV morphine every 30 min as needed on the surgical ward until the NRS score was < 4 and were required to stay in the hospital for 2 to 3 days. If PONV occurred, the patient was treated with IV tropisetron (4.48 mg) and IV metoclopramide (10 mg).
After block completion, the sensorimotor blockade was evaluated every 5 min for 30 min by a blinded investigator. The motor blockade was evaluated using shoulder abduction of the axillary nerve with a validated 3-point scale: 0 = no block, 1 = paresis, and 2 = paralysis. The external rotation lag sign (ERLS) test [20] modified to the lateral position based on Aliste et al.’s study [21] was used to evaluate the motor blockade of the SSN. Pre-block assessments with the ERLS test were performed for all patients, and infraspinatus muscle strength was assessed to evaluate SSN motor function. Quantification of the evaluation results was also based on a 3-point scale: 0 = no block, normal pre-block ERLS test results; 1 = paresis, patient could hold position against gravity but could not sustain external resistance; and 2 = paralysis, patient was completely unable to hold position against gravity. Similarly, the sensory blockade was evaluated in the cutaneous area overlying the clavicle for the supraclavicular nerve and lateral surface of the deltoid for the axillary nerve. Each territory was also graded on a 3-point scale using the pinprick test [18]: 0 = normal, 1 = loss of sensation to pinprick, and 2 = loss of sensation to light touch. A score of 2 points indicated complete sensory or motor blockade of each innervated nerve. The primary outcome was the proportion of patients with complete motor blockade of the SSN 20 min after the injection. The maximal composite score of the sensory score (axillary and supraclavicular nerves) and motor score (suprascapular and axillary nerves) was eight points. A minimal composite score of 7 points was considered satisfactory sensory-motor blockade.
Diaphragmatic excursion was measured in M-mode before the nerve block and 35 min after injection, as described by Boussuges et al. [22] and Renes et al. [23], respectively. When patients inhaled rapidly through their nose (sniff) in the supine position, they were scanned along the anterior axillary line at the level of the costal margin with the cranial angle of the probe. A ≥ 75% reduction in diaphragmatic excursion (complete) or paradoxical movement was defined as HDP, a 25%–75% reduction was defined as partial diaphragmatic paralysis, and a < 25% reduction was defined as absent diaphragmatic paralysis.
The secondary outcomes were as follows. The performance time was defined as the interval from the moment that the ultrasound probe contacted the skin until the moment the needle was fully removed after injection. The skin around the superior, anterior, lateral, and posterior incisions for arthroscopic shoulder surgery was tested for anesthesia using forceps at 40 min. Successful surgical anesthesia was defined as no movement in response to the skin incision, insertion of the blunt trocar, and subsequent intra-articular manipulation. The research assistants also recorded demographic data, including type of surgery; surgical duration (defined as the interval from skin incision to closure); propofol consumption; and block-related complications including vascular puncture, pneumothorax, LA toxicity, Horner’s syndrome, hoarse voice, dyspnea, and hypoxemia (≤ 92%) during surgery. In addition, pain scores at rest at 12 and 24 h post-operation were evaluated using the NRS. Patient satisfaction with the anesthesia method was graded on a 5-point scale: 1 = very dissatisfied, 2 = dissatisfied, 3 = neutral, 4 = satisfied, 5 = very satisfied, and assessed at 24 h post-operation. The duration of the sensory blockade was defined as the interval between block completion and the patient reporting that the affected limb felt normal compared with the contralateral limb. The duration of the motor blockade was defined as the interval between block completion and a return to pre-surgical hand grip strength. Any symptoms suggestive of nerve injury, such as persistent paresthesia or weakness in the upper limb, were recorded during the follow-up telephone call 7 days after surgery.
We expected that a satisfactory percentage of patients receiving CCBs would have complete motor blockade of the SSN along with a reduction in the incidence of HDP. However, no data for CCBs were available regarding these variables. Therefore, we conducted a pilot study with 15 patients per group, none of which were included in this study. The proportion of patients with complete motor blockade of the SSN was 93.3% (14/15, CCB) vs. 86.7% (13/15, ISB) at 20 min after injection, and the incidence of complete and partial HDP was 0% vs. 100% for the CCB and ISB groups at 35 min after injection, respectively. We assumed that a difference in the proportion with complete motor blockade of the SSN between groups of less than −5% was considered non-inferior. We calculated a sample size of 104 patients per group to provide a statistical power of 0.80 and a one-sided 97.5% CI. However, for HDP, fewer than 10 patients were needed to account for a superiority test. Finally, we recruited 232 patients to account for a possible 10% dropout rate, based on non-inferiority study designs.
Statistical analyses were performed using SPSS for Windows 21.0 (SPSS Inc., USA). Normality of the data was verified using the Kolmogorov-Smirnov test. Continuous variables were described as the mean ± SD or median (Q1, Q3), as appropriate, and categorical variables were described as the number (percentage). Continuous parametric and non-parametric data were analyzed using the independent samples t-test and Mann-Whitney U test, respectively. Categorical and dichotomous data were compared using the chi-square test or Fisher’s exact test, as appropriate. Statistical significance was set at P < 0.05.
A total of 232 patients were recruited for our study between December 2020 and December 2021, 20 of which were excluded because they did not meet the inclusion criteria, they refused to participate, or the surgical procedure was changed (Fig. 1). In total, 212 patients (106 in each group) completed the study and were included in the final analysis. Patient and surgical characteristics were comparable between the groups (Table 1).
The ISB and CCB were successfully performed in both groups. For the primary outcome, 66% of patients in the ISB group vs. 53% in the CCB group had complete motor blockade of the SSN (P = 0.069) 20 min after injection (Fig. 2A). The absolute difference between the groups was 13% (95% CI [0, 0.26]), which exceeded the predefined non-inferiority margin of −5%. In addition, the CCB resulted in a lower incidence of HDP at 35 min after injection, with 0 vs. 70 (66%) patients developing complete HDP, 8 (7.5%) vs. 28 (26.4%) developing partial HDP, and 98 (92.5%) vs. 8 (7.5%) developing no HDP in the CCB and ISB groups, respectively (P < 0.001) (Fig. 2B).
Details regarding the characteristics of the sensorimotor blockade after the nerve block are presented in Figs. 2 and 3. Significantly more patients had complete motor blockade of the SSN at 25 min (ISB 85% vs. CCB 73%, P = 0.043). However, no differences were found during the first 20 or 30 min (Fig. 2A). The ISB group had a higher proportion of patients with complete sensory function of the axillary nerve at 20 min (100% vs. 94%, P = 0.029) and motor blockade at 15 min (57% vs. 38%, P = 0.009). However, no differences in the other measurement intervals were found between the groups (Figs. 3A and B). During the first 30 min, the proportion of patients with a complete sensory blockade of the supraclavicular nerve at all measurement intervals was comparable between the two groups (all P > 0.05) (Fig. 3C). Additionally, the two groups had a comparable proportion of patients with complete sensory-motor blockade, meaning a minimal composite score of 7 points from the three nerves (axillary and supraclavicular nerves and SSN) (Fig. 3D).
The block-related and perioperative outcomes between the groups are shown in Table 2. The performance time was slightly longer in the CCB group than in the ISB group (P = 0.047). None of the patients in either group had a failed block, developed hypoxemia, or required a rescue block or conversion to GA. Statistically significant differences were seen between the groups in terms of the incidence of Horner’s syndrome and dyspnea (all P < 0.05) but not in terms of hoarse voice. The duration of the sensory or motor blockade, incidence of rescue analgesics, propofol consumption, NRS score 12 and 24 h post-operation, PONV within 24 h, and patient satisfaction score were similar between the groups. No neurological deficits were noted at the follow-up 7 days after surgery.
The ISB is recognized as an effective anesthetic and analgesic for shoulder surgery, but it impairs diaphragmatic function to varying degrees [1,9]. At the level of the cords, the axillary and subscapular nerves arise from the posterior cord, while the lateral pectoral nerve arises from the lateral cord [24]. The CCB is performed in the costoclavicular space where these three cords are tightly clustered together, allowing for a single injection to provide a complete constant blockade of the three cords [25]. However, whether the CCB could be used as an alternative approach to the ISB to block the SSN was not fully clear [15,21]. The SSN and axillary nerves provide major sensorimotor innervation of the shoulder, as the former arises from the upper trunk of the brachial plexus and starts to diverge at the proximal supraclavicular region [24,26,27]. We failed to demonstrate the non-inferiority of the CCB compared to the ISB in terms of complete motor blockade of the SSN 20 min after injection. However, the proportion of patients with complete motor blockade of the SSN at 30 min was comparable between the groups. In a study by Koyyalamudi et al. [12], a single cadaveric injection in the costoclavicular space showed that the dye consistently spread cephalad and stained the SSN and all three cords while sparing the phrenic nerve. In a clinical study, a single-injection CCB provided an analgesic effect equivalent to that of the ISB for shoulder surgery [10]. Furthermore, a retrograde channel between the costoclavicular and supraclavicular spaces likely facilitated the cephalad spread of LA, as described in a previous study [13]. Therefore, a single-injection CCB has considerable potential to achieve blockade of all the important nerves innervating the shoulder joint and may serve as a promising alternative approach to the ISB in arthroscopic shoulder surgery.
The results of our study support the use of the CCB as the sole anesthesia technique for arthroscopic shoulder surgery, but further careful evaluation of the sensorimotor blockade of the innervated nerves of the shoulder before surgery and anesthetization of the skin area innervated by the supraclavicular nerve are necessary. Clinical data on the block characteristics of the SSN blockade after a supraclavicular block are not available. However, supraclavicular blocks have been used for anesthesia during arthroscopic shoulder surgery [9]. In addition, because multiple intraplexus septa exist in the supraclavicular fossa, whether the injection techniques will affect the success rate of the SSN blockade requires further study. Notably, the rates of complete motor blockade of the SSN by the CCB were similar to those by the ISB at all other predetermined intervals (except at 25 min) in this study, as well as the sensory and/or motor blockade of the axillary and supraclavicular nerves at 25–30 min. Given the similarity of the block dynamics between the two approaches, including the sensorimotor blockade of the individual nerves and block duration, we believe that the results of this study show the benefits and safety of enhanced recovery after surgery for ambulatory shoulder surgery using single-injection CCBs [28].
The existence of a paraneural sheath and intraplexus septum between the three cords has been reported in both cadavers and in vivo [29,30] and are believed to reduce injectate spread to the surrounding cords and toward the proximal trajectory of the brachial plexus. The paraneural sheath, visualized as a hyperechoic fascial layer surrounding the cords of the brachial plexus, may serve as part of the “axillary tunnel” as a conduit for the spread of the LA to the supraclavicular space during CCBs [31]. In addition, an intraplexus septum splits the subparaneural compartment into two fascial compartments [25]. This hyperechoic connective tissue surrounding the brachial plexus is also visualized using a classical or intertruncal approach to the supraclavicular block [32]. Multipoint injection techniques display a shorter onset time in patients undergoing upper limb surgery, but it remains unknown whether the diffusion distance of the LA is influenced by separate injections in different fascial compartments for shoulder surgery [33]. Due to the cephalad spread, distal approaches to the brachial plexus block seem to be more important when applied in shoulder surgery.
In previous studies [10,12], a single injection of 20 ml of LA was slowly administered into the center of the three cords. The results of our study were consistent with those of other studies that have similarly examined the analgesic effect and rate of phrenic nerve paralysis for the CCB. Postoperative pain scores in both groups were equivalent within 24 h post-operation. Similarly, the CCB was associated with a significantly lower incidence of HDP (absent, 98 vs. 8 patients; partial, 8 vs. 28 patients; complete, 0 vs. 70 patients). The incidences of Horner’s syndrome and patient-reported dyspnea were much lower in the CCB group than in the ISB group. In contrast to Aliste et al. [10]’s study, none of the patients had HDP 30 min after the blocks or in the PACU. This difference could be explained by the relatively small sample size (22 patients in Aliste et al.’s study vs. 106 patients in our study). Moving further caudally (e.g., paracoracoid approach to infraclavicular or retroclavicular block) [9,34,35] may further lower the risk of HDP, while displaying inferior postoperative analgesia compared to the ISB or a higher incidence of an incomplete blockade of the suprascapular and/or axillary nerves. As a result, potential anatomical causes may explain why the costoclavicular space is a suitable location for anesthetizing all innervated nerves of the shoulder, while sparing the phrenic nerve. In other words, the paraneural sheath and fascial compartments surrounding the cords play a crucial role in the spread of LA, though this is a double-edged sword. These connective tissues and all three cords are clustered together to form a potential retrograde channel, forcing LA to spread around and cephalad. On the other hand, increasing the distance between the costoclavicular space and the interscalene groove results in a physical barrier that obstructs the spread of LA to the phrenic nerve, stellate ganglion, or recurrent laryngeal nerve.
Previous studies have confirmed that for patients undergoing arthroscopic shoulder surgery, the ISB has benefits, including quicker recovery, opioid sparing effects, intraoperative hemodynamic variability, improved patient satisfaction, and reduced GA-related side effects [1,7]. In recent years, a renewed interest in diaphragm-sparing nerve blocks in combination with GA for shoulder surgery have surfaced [36]. Different blocks (e.g., axillary, paracoracoid, costoclavicular, and supraclavicular) in addition to the option of the SSN for perioperative analgesia have been proposed, in which proximal approaches (at the level of the clavicle) are more likely to result in analgesic effects similar to those of the ISB [37–39]. In a patient with normal anatomy, the shoulder receives sensorimotor innervation from the axillary, lateral pectoral, and subscapular nerves. We decided to err on the side of caution in this study and highlighted motor blockade of the SSN as the primary outcome since it is the most difficult for LA spread to reach during a CCB [24]. Interestingly, the block dynamics of single-injection CCBs were similar to those of ISBs 30 min after injection. A reliable CCB could anesthetize all of these nerves for arthroscopic shoulder surgery while eliminating block-related complications to a greater extent. In this study, all patients received a single mode brachial plexus block under monitored anesthesia care, and none required conversion to GA because of incomplete blocks or hypoxemia. However, ultrasound-guided CCBs are associated with slightly longer performance times. The cramped space for adjusting the direction of the needle may contribute to this. Nevertheless, we recommend ultrasound-guided CCBs be considered as an alternative approach to the ISB for anesthesia in arthroscopic shoulder surgery, especially in patients with pre-existing pulmonary pathology.
This study had some limitations. First, we chose to perform a single-injection CCB for surgical anesthesia, which was bold. Although, in theory, it should work as well as an ISB with appropriate sedation and close monitoring, this has yet to be formally shown in clinical practice. In addition, we deliberately chose to evaluate the sensory and/or motor blockade of the suprascapular and axillary nerves because of the important roles they play in shoulder joint innervation. A double evaluation of the axillary nerve (sensory and motor) was performed to confirm the effect of the nerve block at the cord level. All patients were placed in the lateral decubitus position during surgery. However, the beach chair position may not only cause more intraoperative discomfort to the patients but also make achieving a complete and safe intraoperative block more challenging [40]. Further studies are required to confirm these findings. Second, we used a total LA volume of 20 ml in both groups. This volume is likely to be relatively high for ultrasound-guided ISBs. A larger LA volume is usually associated with HDP, Horner’s syndrome, recurrent laryngeal nerve palsy, and superficial cervical plexus block. However, a larger LA volume may shorten the time required for complete motor blockade of the SSN with the CCB. Subsequently, a study aimed at determining an optimal injectate volume for both approaches would help to control the associated bias. Third, we performed a single-injection technique between the three cords of the brachial plexus. It is unclear whether multiple injection sites within the costoclavicular space could influence the spread of the LA, particularly the persistent blocking effect of the SSN. Future studies are needed to evaluate the effect of various injection volumes or multiple injection techniques in the costoclavicular space on block dynamics. Fourth, the blocks were performed by experienced anesthesiologists who had extensive experience with CCBs (over 60 attempts) before this study. This protocol needs to be carried out carefully by novices or anyone unfamiliar with the CCB.
In conclusion, compared with the ISB, we failed to demonstrate the non-inferiority of complete motor blockade of the SSN for the CCB, but achieved similar block characteristics at 30 min while reducing the risk of HDP. Additionally, compared with the single-injection ISB, satisfactory surgical anesthesia, a lower incidence of block-related complications, and equally effective analgesia was seen with the CCB. These results demonstrated that ultrasound-guided CCBs may be a more appropriate choice for patients with impaired lung function undergoing arthroscopic shoulder surgery. Further trials are required to investigate the optimal volume, multiple injection techniques, and continuous catheter analgesia for arthroscopic shoulder surgeries.
Notes
Funding
This work was supported by Science and Technology Projects in Guangzhou (Grant No. 202102010494) and the Medical Scientific Research Foundation of Guangdong Province of China (Grant No. A2022018 and A2021047).
References
1. Bosco L, Zhou C, Murdoch JA, Bicknell R, Hopman WM, Phelan R, et al. Pre- or postoperative interscalene block and/or general anesthesia for arthroscopic shoulder surgery: a retrospective observational study. Can J Anaesth. 2017; 64:1048–58.

2. Ardon AE, Prasad A, McClain RL, Melton MS, Nielsen KC, Greengrass R. Regional anesthesia for ambulatory anesthesiologists. Anesthesiol Clin. 2019; 37:265–87.

3. Yan S, Zhao Y, Zhang H. Efficacy and safety of interscalene block combined with general anesthesia for arthroscopic shoulder surgery: a meta-analysis. J Clin Anesth. 2018; 47:74–9.

4. Hadzic A, Williams BA, Karaca PE, Hobeika P, Unis G, Dermksian J, et al. For outpatient rotator cuff surgery, nerve block anesthesia provides superior same-day recovery over general anesthesia. Anesthesiology. 2005; 102:1001–7.

5. D’Alessio JG, Rosenblum M, Shea KP, Freitas DG. A retrospective comparison of interscalene block and general anesthesia for ambulatory surgery shoulder arthroscopy. Reg Anesth. 1995; 20:62–8.
6. Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009; 103:428–33.

7. Hussain N, Goldar G, Ragina N, Banfield L, Laffey JG, Abdallah FW. Suprascapular and interscalene nerve block for shoulder surgery: a systematic review and meta-analysis. Anesthesiology. 2017; 127:998–1013.
8. Holbrook HS, Parker BR. Peripheral nerve injury following interscalene blocks: a systematic review to guide orthopedic surgeons. Orthopedics. 2018; 41:e598–606.

9. Tran DQ, Elgueta MF, Aliste J, Finlayson RJ. Diaphragm-sparing nerve blocks for shoulder surgery. Reg Anesth Pain Med. 2017; 42:32–8.

10. Aliste J, Bravo D, Layera S, Fernandez D, Jara A, Maccioni C, et al. Randomized comparison between interscalene and costoclavicular blocks for arthroscopic shoulder surgery. Reg Anesth Pain Med. 2019; 44:472–7.

11. Karmakar MK, Sala-Blanch X, Songthamwat B, Tsui BC. Benefits of the costoclavicular space for ultrasound-guided infraclavicular brachial plexus block: description of a costoclavicular approach. Reg Anesth Pain Med. 2015; 40:287–8.
12. Koyyalamudi V, Langley NR, Harbell MW, Kraus MB, Craner RC, Seamans DP. Evaluating the spread of costoclavicular brachial plexus block: an anatomical study. Reg Anesth Pain Med. 2021; 46:31–4.

13. Garcia-Vitoria C, Vizuete J, Lopez Navarro AM, Bosch M. Costoclavicular space: a reliable gate for continuous regional anesthesia catheter insertion. Anesthesiology. 2017; 127:712.
14. Sonawane K, Mistry T. Expanding the horizon of costoclavicular block - shouldering new responsibility! Braz J Anesthesiol. 2021; 71:193–4.

15. Hewson DW, Oldman M, Bedforth NM. Regional anaesthesia for shoulder surgery. BJA Educ. 2019; 19:98–104.

16. Kwon W, Lee SM, Bang S. Costoclavicular block for shoulder surgery in a patient with tracheobronchopathia osteochondroplastica and COPD. J Clin Anesth. 2019; 55:13–4.

17. Subramanian VK, Mistry T, Balasubramanian S, Palanichamy G. Combination of costoclavicular and upper trunk (CUT) block for the surgical repair of proximal humerus fracture in a severely obese patient. J Clin Anesth. 2021; 74:110396.

18. Kang R, Jeong JS, Chin KJ, Yoo JC, Lee JH, Choi SJ, et al. Superior trunk block provides noninferior analgesia compared with interscalene brachial plexus block in arthroscopic shoulder surgery. Anesthesiology. 2019; 131:1316–26.

19. Maybin J, Townsley P, Bedforth N, Allan A. Ultrasound guided supraclavicular nerve blockade: first technical description and the relevance for shoulder surgery under regional anaesthesia. Anaesthesia. 2011; 66:1053–5.

20. Hertel R, Ballmer FT, Lombert SM, Gerber C. Lag signs in the diagnosis of rotator cuff rupture. J Shoulder Elbow Surg. 1996; 5:307–13.

21. Aliste J, Cristi-Sanchez I, Bermudez L, Layera S, Bravo D, Tran Q. Assessing surgical anesthesia for shoulder surgery. Reg Anesth Pain Med. 2020; 45:675–6.

22. Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009; 135:391–400.

23. Renes SH, Rettig HC, Gielen MJ, Wilder-Smith OH, van Geffen GJ. Ultrasound-guided low-dose interscalene brachial plexus block reduces the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med. 2009; 34:498–502.

24. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014; 27:210–21.

25. Li JW, Songthamwat B, Samy W, Sala-Blanch X, Karmakar MK. Ultrasound-guided costoclavicular brachial plexus block: sonoanatomy, technique, and block dynamics. Reg Anesth Pain Med. 2017; 42:233–40.
26. Fossati Puertas S, Lopez Medina ME, Sante Serna L. Anaesthesia for arthroscopic shoulder surgery, do we have alternatives to the interescalene block? Rev Esp Anestesiol Reanim (Engl Ed). 2019; 66:406–7.

27. Borgeat A, Ekatodramis G. Anaesthesia for shoulder surgery. Best Pract Res Clin Anaesthesiol. 2002; 16:211–25.

28. Grosh T, Elkassabany NM. Enhanced recovery after shoulder arthroplasty. Anesthesiol Clin. 2018; 36:417–30.

29. Monzo E, Boezaart AP, Tubbs RS, Sanroman-Junquera M, Nin OC, Reina MA. A reliable septum exists between the lateral cord and medial and posterior cords in the costoclavicular region: clinical and microanatomical considerations in brachial plexus anesthetic blockade. Clin Anat. 2021; 34:411–9.

30. Areeruk P, Karmakar MK, Reina MA, Mok LY, Sivakumar RK, Sala-Blanch X. High-definition ultrasound imaging defines the paraneural sheath and fascial compartments surrounding the cords of the brachial plexus at the costoclavicular space and lateral infraclavicular fossa. Reg Anesth Pain Med. 2021; 46:500–6.

31. Sala-Blanch X, Reina MA, Pangthipampai P, Karmakar MK. Anatomic basis for brachial plexus block at the costoclavicular space: a cadaver anatomic study. Reg Anesth Pain Med. 2016; 41:387–91.
32. Siddiqui U, Perlas A, Chin K, Reina MA, Sala-Blanch X, Niazi A, et al. Intertruncal approach to the supraclavicular brachial plexus, current controversies and technical update: a daring discourse. Reg Anesth Pain Med. 2020; 45:377–80.

33. Layera S, Aliste J, Bravo D, Fernandez D, Garcia A, Finlayson RJ, et al. Single- versus double-injection costoclavicular block: a randomized comparison. Reg Anesth Pain Med. 2020; 45:209–13.

34. Georgiadis PL, Vlassakov KV, Patton ME, Lirk PB, Janfaza DR, Zeballos JL, et al. Ultrasound-guided supraclavicular vs. retroclavicular block of the brachial plexus: comparison of ipsilateral diaphragmatic function: a randomised clinical trial. Eur J Anaesthesiol. 2021; 38:64–72.

35. Gianesello L, Pavoni V, Burzio I, Boccaccini A. Respiratory effect of interscalene brachial plexus block vs combined infraclavicular plexus block with suprascapular nerve block for arthroscopic shoulder surgery. J Clin Anesth. 2018; 44:117–8.

36. Cubillos J, Giron-Arango L, Munoz-Leyva F. Diaphragm-sparing brachial plexus blocks: a focused review of current evidence and their role during the COVID-19 pandemic. Curr Opin Anaesthesiol. 2020; 33:685–91.

37. Aliste J, Bravo D, Fernandez D, Layera S, Finlayson RJ, Tran DQ. A randomized comparison between interscalene and small-volume supraclavicular blocks for arthroscopic shoulder surgery. Reg Anesth Pain Med. 2018; 43:590–5.

38. Aliste J, Bravo D, Finlayson RJ, Tran DQ. A randomized comparison between interscalene and combined infraclavicular-suprascapular blocks for arthroscopic shoulder surgery. Can J Anaesth. 2018; 65:280–7.

39. Neuts A, Stessel B, Wouters PF, Dierickx C, Cools W, Ory JP, et al. Selective suprascapular and axillary nerve block versus interscalene plexus block for pain control after arthroscopic shoulder surgery: a noninferiority randomized parallel-controlled clinical trial. Reg Anesth Pain Med. 2018; 43:738–44.
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of patient selection. ISB: interscalene block, CCB: costoclavicular block.
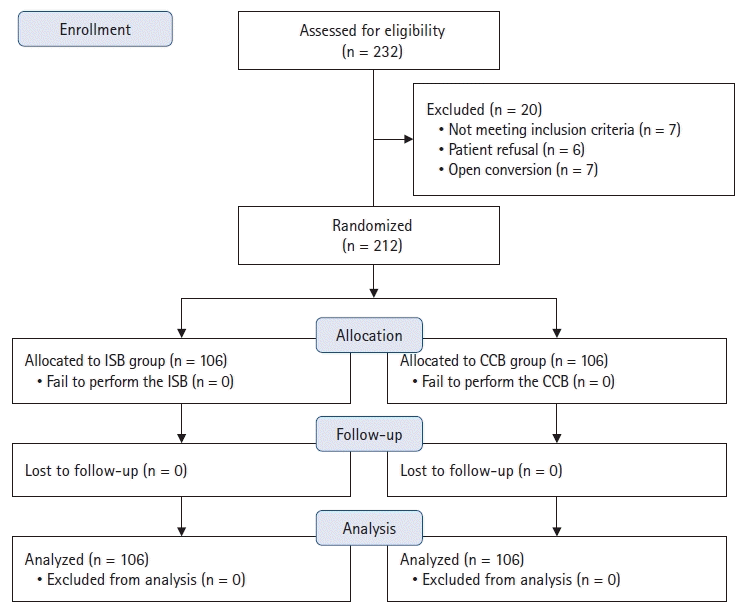
Fig. 2.
Diagrams show the block characteristics of the SSN and HDP. (A) Percentage of patients with complete motor blockade (score of 2 points) of the SSN at 5-min intervals. (B) Percentage of patients with HDP (complete/partial/absent) at 35 min after injection. SSN: suprascapular nerve, HDP: hemidiaphragmatic paralysis, ISB: interscalene block, CCB: costoclavicular block.
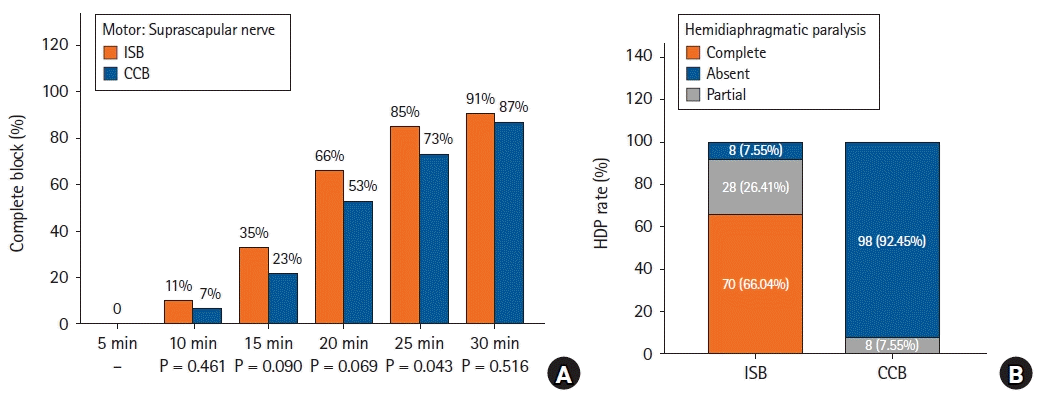
Fig. 3.
Diagrams show the block characteristics of the axillary, supraclavicular nerve, and sensory-motor blockade. (A, B) Percentage of patients with complete sensory or motor blockade of the axillary nerve. (C) Percentage of patients with complete sensory blockade of the supraclavicular nerve. (D) Percentage of patients with complete sensory-motor blockade (a minimal composite score of 7 points) over time. The sensory and/or motor blockade was evaluated every 5 min for 30 min. ISB: interscalene block, CCB: costoclavicular block.
Table 1.
Patients and Surgical Characteristics
Table 2.
Block-related and Perioperative Outcomes




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download