Abstract
Purpose
This phase I study was conducted to determine the maximum tolerated dose and the recommended phase II dose of weekly administered Genexol-PM combined with carboplatin in patients with gynecologic cancer.
Materials and Methods
This open-label, phase I, dose-escalation study of weekly Genexol-PM included 18 patients with gynecologic cancer, who were equally divided into three cohorts of dose levels. Cohort 1 received 100 mg/m2 Genexol-PM and 5 area under the curve (AUC) carboplatin, cohort 2 received 120 mg/m2 Genexol-PM and 5 AUC carboplatin, and cohort 3 received 120 mg/m2 Genexol-PM and 6 AUC carboplatin. The safety and efficacy of each dose were analyzed for each cohort.
Results
Of the 18 patients, 11 patients were newly diagnosed and seven patients were recurrent cases. No dose-limiting toxicity was observed. The maximum tolerated dose was not defined, but a dose up to 120 mg/m2 of Genexol-PM in combination with AUC 5–6 of carboplatin could be recommended for a phase II study. In this intention-to-treat population, five patients dropped out of the study (carboplatin-related hypersensitivity, n=1; refusal of consent, n=4). Most patients (88.9%) with adverse events recovered without sequelae, and no treatment-related death occurred. The overall response rate of weekly Genexol-PM in combination with carboplatin was 72.2%.
Paclitaxel and carboplatin (TC) is a standard treatment for various gynecologic cancers [1–3]. The TC regimen has been reported to elicit an overall response rate (ORR) of 68% to 70% as a first-line treatment in ovarian cancer [4]. The GOG0209 trial reported that TC is not inferior to the paclitaxel-doxorubicin-cisplatin regimen and had a more tolerable toxicity profile, which suggested that TC should be considered as a first-line regimen for advanced or recurrent endometrial cancer [5]. Recently, therapeutic advances have been made in gynecologic cancer including olaparib in BRCA-mutated ovarian cancer and pembrolizumab in mismatch repair-deficient or microsatellite instability-high endometrial cancer [6,7]. However, recent results of targeted therapy and immunotherapy have limitations in that they can only be applied to limited groups of patients with specific molecular characteristics. Therefore, paclitaxel remains one of the most important anticancer drugs for gynecologic cancer.
In order to enhance the antitumor effect of the TC regimen and reduce its toxicity, alternative dosing schedules have been investigated [8]. In the phase III JGOG 3016 trial, dose-dense weekly paclitaxel (80 mg/m2) plus 6 area under the curve (AUC) of carboplatin had a better median progression-free survival (PFS) compared with the tri-weekly conventional regimen in ovarian cancer patients (28.0 vs. 17.2 months) [9,10]. In another phase III study, MITO-7, the combination of weekly paclitaxel (60 mg/m2) and carboplatin (3 AUC) was better tolerated than the tri-weekly regimen, with favorable quality-of-life scores and reduced frequency of grade 3–4 toxicities [11]. Based on these findings, it was presumed that paclitaxel could be administered on a weekly dosing schedule in combination with carboplatin, which might lead to better tumor response and lower toxicity than the tri-weekly administration.
To increase the solubility of hydrophobic paclitaxel, paclitaxel has been formulated in a micelle-forming vehicle, Cremophor EL (CrEL) [12]. However, the addition of CrEL was reported to be associated with hypersensitivity reactions and neurotoxicity [13]. Even with an appropriate premedication, CrEL-paclitaxel formulations are known to induce mild hypersensitivity reactions in ~44% of patients and grade 3/4 reactions in ~2% of patients [14]. To overcome these problems, CrEL-free paclitaxel formulations have been developed to reduce the dose-limiting toxicities (DLTs) and to increase the antitumor activity. Genexol-PM (Samyang Co., Seoul, Korea) is a novel formulation of polymeric micellar paclitaxel using nontoxic and biodegradable diblock copolymers as a solubilizer. An in vivo study showed that the antitumor activity of Genexol-PM was superior to that of conventional paclitaxel, with a 3-times higher maximum tolerated dose (MTD) [15].
Accordingly, phase I and II trials were conducted using tri-weekly Genexol-PM plus carboplatin in patients with epithelial ovarian cancer [13,16]. In the phase I study, despite the administration of a higher paclitaxel dose with Genexol-PM, paclitaxel-related adverse events were comparable to those with conventional paclitaxel [13]. The phase II study reported that the efficacy of tri-weekly Genexol-PM plus carboplatin was not inferior to that of the standard paclitaxel regimen, with tolerable toxicity profiles [16]. However, there have been no studies that investigated the safety and tolerability of weekly Genexol-PM in patients with gynecologic cancer. In the present phase I study, we examined the MTD and the recommended phase II dose (RP2D) of weekly Genexol-PM administered in combination with carboplatin in patients with gynecologic cancer.
This open-label, phase I, dose-escalation study in patients with gynecologic cancer was performed at Asan Medical Center (Seoul, Korea).
Patients were eligible for inclusion in the study when they met all of the following criteria: (1) between 20 and 80 years of age; (2) newly diagnosed or relapsed gynecologic cancer (i.e., epithelial ovarian cancer, fallopian tube cancer, primary peritoneal cancer, cervical cancer, or uterine corpus cancer); (3) appropriate for paclitaxel and carboplatin combination therapy (in the case of recurrence, only platinum-sensitive diseases were included); (4) Eastern Cooperative Oncology Group (ECOG) performance grade 0–2; (5) adequate organ function and hematological status; (6) written informed consent provided before participation.
Exclusion criteria of the study included the following: (1) history of carcinoma other than gynecologic cancer in the past 5 years; (2) received radiotherapy in the pelvis or abdominal cavity; (3) received hormone therapy or immunotherapy for gynecologic cancer; (4) received a major operation other than debulking for gynecologic cancer within 2 weeks before the screening; (5) history of central nervous system metastases; (6) National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE ver. 4.0) grade 1 or more sensory or motor neuropathy; (7) preexisting uncontrolled comorbidities, including psychiatric illness, active infectious disease, severe cardiovascular disease, or hypersensitivity to any of the study drugs or the vehicle. The complete criteria of eligibility are provided in the Supplementary Material.
Patients were divided into 3 cohorts according to the Genexol-PM dose: cohort 1 (100 mg/m2), cohort 2 (120 mg/m2), and cohort 3 (120 mg/m2). Carboplatin was administered at a dose of 5 AUC in cohorts 1 and 2 and 6 AUC in cohort 3. The two carboplatin doses were established according to the National Comprehensive Cancer Network guidelines [1–3]. Genexol-PM was administered on days 1, 8, and 15 of each 21-day cycle and carboplatin was administered on day 1 of each cycle for a maximum of 6 cycles. For responsive patients, the treatment duration was extended at the investigator’s discretion. When subjects experienced a DLT, they received the next lower dose; when a subject continued to exhibit toxicity after dose lowering, the drug was withdrawn.
Each cohort included six patients and their DLTs were evaluated for each cycle. When one or less patient had DLTs, the following six patients were enrolled in the next dose cohort. The study was planned to stop dose escalations when two or more patients experienced DLTs in any cohort. The dose just below the lowest level at which two or more patients exhibit DLTs would be determined as the MTD. This dose would be defined as the RP2D, based on general toxicity. If DLTs were not reported in two or more patients in cohort 3, the dose of 120 mg/m2 or less would be identified as the RP2D based on the adverse events (Fig. 1).
The primary objective of the study was to evaluate the safety and tolerability of weekly Genexol-PM in patients with gynecologic cancers and to define the relevant MTD and RP2D. The secondary objective was to assess the antitumor response of weekly Genexol-PM.
Adverse events were assessed at each visit and the severities of adverse events were reported according to CTCAE v4.0. Adverse drug reactions (ADRs) and serious adverse events (SAEs) were reported separately. SAEs were defined as any adverse events that resulted in (1) prolongation of hospital stay, (2) persistent or significant disability or incapacity, (3) congenital anomaly or birth defect, or (4) death or life-threatening situations. DLTs were defined as any of the following events: (1) grade 4 neutropenia for more than a week or febrile neutropenia despite the administration of granulocyte-colony stimulating factor; (2) grade 4 thrombocytopenia for more than a week or grade 3 thrombocytopenia with active bleeding; (3) grade 3 or higher non-hematologic toxicity, except for nausea and vomiting; and (4) grade 3 or higher hypersensitivity despite premedication.
The antitumor response of weekly Genexol-PM plus carboplatin was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST) v1.0 [17] and the Gynecologic Cancer Intergroup (GCIG) criteria. For patients with measurable lesions, the overall responses (complete response [CR] or partial response [PR]) were evaluated after every 2 cycles by a radiologist according to RECIST v1.0. For all patients, tumor responses were also evaluated using the carbohydrate antigen 125 (CA-125) response per the GCIG criteria [18]. The ORR was defined as the proportion of patients who had a CR or PR per the RECIST criteria or the CA-125 response per the GCIG criteria. PFS was defined as the duration between the first date of weekly Genexol-PM and the first disease progression or death, and overall survival (OS) was defined as the duration between the first date of weekly Genexol-PM and death.
Patient characteristics and safety evaluations were summarized descriptively. The best ORR was defined as the proportion of patients who had a CR or PR per RECIST criteria or the CA-125 response per GCIG criteria. The 95% confidence intervals of ORRs were calculated using the Clopper-Pearson method. Survival curve analysis for PFS and OS was performed using the Kaplan-Meier method. We used SPSS Statistics for Windows, ver. 21 (IBM Corp., Armonk, NY) and SAS ver. 9.4 (SAS Institute, Cary, NC) for all statistical analyses.
The characteristics of the study patients are presented in Table 1. Between April 12, 2016 and February 13, 2017, 18 patients were enrolled in this study and assigned to 3 sequential dose cohorts. These 18 patients were included in the intention-to-treat (ITT) population because all of them received Genexol-PM at least once. Except for one patient who withdrew consent after cycle 1, the tumor responses of 17 patients were evaluated. Among the ITT population, 11 patients were newly diagnosed and seven patients were relapsed cases. All recurrent cases had platinum-sensitive first recurrent diseases and had no history of chemotherapy after recurrence. The origin of cancer was ovary and fallopian tube in 15 cases and endometrium in three cases. All of the endometrial cancer patients were newly diagnosed. Among the ITT population, five patients dropped out of the study due to carboplatin-related hypersensitivity (n=1) or refusal of consent (n=4); the remaining 13 patients completed the study and constituted the per-protocol (PP) population. The reason four patients withdrew their consent for the study was the inconvenience of weekly treatment. The median age was 56.0 years (range, 36 to 68 years). At screening, all patients had ECOG PS 0. Of the 18 treated patients, the histopathological grade of the tumors was grade 3 in 13 patients, grade 2 in three patients, grade 1 in one patient, and unknown in one patient. The patient with stage IA disease at diagnosis was a recurrent case and had an abdominal wall metastasis.
The doses of Genexol-PM and carboplatin for each group and the number of cycles administered are shown in Table 2. A total of five, five, and three patients in cohorts 1, 2, and 3 completed the treatment, respectively. No DLT was observed in all cohorts, and the MTD for Genexol-PM was not reached. Therefore, the RP2D of Genexol-PM was found to be 120 mg/m2 or less.
Throughout the study period, a total of 377 adverse events were recorded, including events unrelated to the study drugs. The incidence rates of adverse events according to the type are as follows: hematologic disorders (267 cases in 18 [100.0%] patients), dermatologic disorders (56 cases in 18 [100.0%] patients), gastrointestinal disorders (32 cases in 15 [83.3%] patients), neurological disorders (12 cases in 9 [50.0%] patients), and immunological disorders (11 cases in 8 [44.4%] patients). Among the adverse events, 41.2% were grade 3 or 4. Treatment was temporarily interrupted in one patient and permanently discontinued in another patient. The patient who discontinued the treatment experienced drug-induced hypersensitivity, which was found to be due to carboplatin. Genexol-PM dose reduction occurred in one patient due to elevated ALT levels. Of the 377 adverse events, 367 (97.3%) cases were resolved without sequelae, and treatment-related death did not occur.
The ADRs reported in each dose cohort are summarized in Table 3. A total of 327 ADRs occurred in 18 patients (100.0%). Neutropenia was the most common hematological ADR, and grade 3/4 neutropenia was observed in 18 patients (100.0%). There were no cases of febrile neutropenia. Grade 3/4 anemia occurred in six patients (33.3%) and grade 3/4 thrombocytopenia was reported in one patient (5.6%). The most common non-hematological ADRs were alopecia (17 [94.4%] patients), followed by pruritus (9 [50.0%]), increased aspartate aminotransferase (8 [44.4%]), and peripheral sensory neuropathy (8 [44.4%]). Among the non-hematological ADRs, there were no episodes that were grade 3 or higher.
Table 4 shows the four cases of SAEs that occurred in two patients during the study. These two patients were each in cohort 1 and cohort 2. The patient in cohort 1 required prolonged hospital stay and granulocyte-colony stimulating factor (G-CSF; filgrastim) treatment due to grade 4 neutropenia. The other patient in cohort 2 reported two events of grade 3/4 neutropenia treated by the administration of G-CSF in hospitalization. Additionally, this patient in cohort 2 reported one event of pneumonia that required prolonged hospital stay and antibiotic therapy. In cohort 3, no SAEs were reported. All SAEs in cohort 1 and cohort 2 were eventually recovered within 4 days.
Of the total participants, six patients had measurable lesions for which antitumor responses could be assessed. One of the patients with measurable lesions withdrew consent after cycle 1 and could not be assessed for treatment response, and the remaining five patients achieved objective responses (3 CRs and 2 PRs). Tumor re-evaluations were performed in these five patients with measurable lesions who had undergone 2 or more cycles of chemotherapy. The results of re-evaluations performed every 2 cycles of chemotherapy did not show notable changes. Based on CA-125, 11 of the 18 subjects in the ITT population achieved treatment response (Table 5).
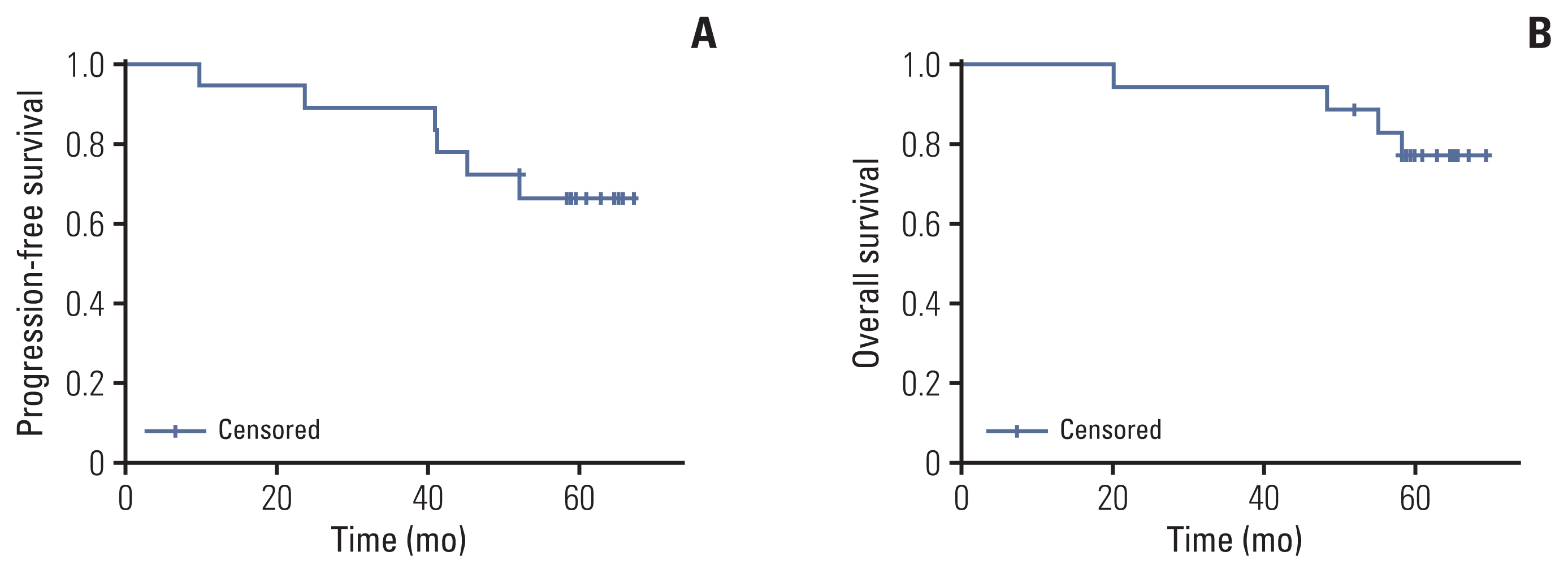
With the combination of RECIST and CA-125 criteria, the ORR was estimated as 72.2% (95% confidence interval [CI], 46.5 to 90.3) in the ITT population. The ORR per dose was 83.3% (95% CI, 35.9 to 99.6) in cohorts 1 and 2 and 50.0% (95% CI, 11.8 to 88.2) in cohort 3. The ORRs per disease state were 72.7% in patients with newly diagnosed diseases and 71.4% in patients with recurrent diseases (S1 Table). The ORR in the PP population was 84.6% (11 of 13 patients). Data cutoff time was February 14, 2022 and the median duration of follow-up was 60.5 months (range, 20.1 to 69.5 months). The median PFS and OS were not reached at data cutoff (Fig. 2).
In this phase I study, we found that weekly Genexol-PM combined with carboplatin had a tolerable safety profile in patients with ovarian/fallopian tube cancer or endometrial cancer. Even after dose escalation to 120 mg/m2 Genexol-PM and 6 AUC carboplatin, there were no DLTs and the MTD was not reached. According to these results, the RP2D of weekly Genexol-PM can be defined as up to 120 mg/m2 when combined with carboplatin administered every 3 weeks, which is higher than the conventional regimen as the currently used TC regimen consists of 60–80 mg/m2 conventional paclitaxel and 5–6 AUC carboplatin [10,19,20]. Notably, DLTs did not occur in our study patients despite using higher doses of paclitaxel compared with conventional paclitaxel.
In the present study, the safety profile of weekly Genexol-PM was generally consistent with the tri-weekly regimen, although neutropenia seemed to be more common with weekly Genexol-PM than with the tri-weekly regimen. Compared with the previous phase I and II studies of tri-weekly Genexol-PM in ovarian cancer patients [13,16], there were more cases of neutropenia in our study, which could be explained by the higher doses of weekly Genexol-PM. In addition, it has been suggested that the risk of chemotherapy-induced neutropenia is specifically associated with a history of chemotherapy [21]. While the previous studies on tri-weekly Genexol-PM in ovarian cancer patients only included participants planning to receive first-line chemotherapy, our study also included recurrent cases with a history of chemotherapy. Six patients (33.3%) in this study had previously received platinum-based chemotherapy as first-line treatment and five of them had received conventional paclitaxel, which likely contributed to the high incidence of neutropenia in this study. Although all of the 18 patients experienced grade 4 neutropenia, there were no cases of febrile neutropenia.
In the ITT population, a total of four cases of SAEs in two patients (each in cohort 1 and cohort 2) were reported. Unlike three cases of grade 3/4 neutropenia, which are believed to be strongly related to the study drug, one case of pneumonia was considered by the investigators not to be related to the study drug. All of these SAEs were all resolved within 4 days.
During this study, there were no cases of neuropathy that were grade 3 or higher. Grade 1–2 peripheral neuropathy was reported in eight patients (50.0%), of whom six patients had recovered at the time of data cutoff. This is similar to the results of previous phase I and II studies of tri-weekly Genexol-PM in which grade 3 or higher neuropathy was not reported [13,16]. Peripheral neuropathy can be caused by paclitaxel itself [22]. However, the absence of grade 3 or higher neuropathy in several studies of Genexol-PM in patients with gynecologic cancer could be explained by the fact that the solvent-free paclitaxel formulation provides the advantages of causing less severe neurotoxicity and more rapid recovery compared with conventional paclitaxel [16]. In addition, after the administration of Genexol-PM, hypersensitivity, another known toxicity of CrEL-paclitaxel, was noted in only one patient as grade 2; conversely, carboplatin-related hypersensitivity reactions were observed in two patients. Considering that the incidence of paclitaxel-related hypersensitivity was reported to range from 8% to 45% [23], the relatively lower incidence of hypersensitivity related to Genexol-PM in this study is likely due to the absence of the micelle-forming vehicle, CrEL.
Although this phase I study was not powered or designed to assess antitumor activity, we found that weekly Genexol-PM had durable antitumor activity in patients with ovarian/fallopian tube cancer or endometrial cancer. All five patients with measurable lesions and for whom tumor assessment was possible showed objective responses, including three CRs and two PRs. The ORR in the ITT population was 72.2% based on tumor assessment and the CA-125 response. A previous phase II study reported that the ORR of tri-weekly Genexol-PM was 88.0% as first-line treatment for ovarian cancer [16], and another phase II study found that the ORR of weekly conventional paclitaxel and carboplatin was 62.5% in patients with recurrent ovarian cancer [24]. The ORR of 72.2% in the present study is meaningful considering that more than half of the patients had advanced and recurrent diseases.
This study has several limitations. First, the main limitations were the small sample size (n=18) and heterogeneity of patient characteristics as both newly diagnosed and recurrent cases were included. However, all patients in this study were eligible for paclitaxel and carboplatin combination therapy, and all of the recurrence cases were platinum-sensitive first recurrent diseases. Additionally, 3 patients with endometrial cancer had primary stage III diseases for which systemic chemotherapy with paclitaxel and carboplatin was recommended. Second, a significant number of participants dropped out of the study (5 [27.8%] patients). Lastly, only two doses of weekly Genexol-PM were tested. The reason for the limited selection of doses is that albumin-bound paclitaxel, which is another class of CrEL-free paclitaxel, was also approved at doses of 100 and 120 mg/m2 for the treatment of metastatic non-small cell lung cancer and pancreatic cancer, respectively [25]. One phase I study of weekly Genexol-PM in Asian patients with solid tumors reported that a dose of 100 mg/m2 was found to be safe without grade 3/4 neutropenia [26]. This led to the dose of weekly Genexol-PM to be set at 100 mg/m2 with gemcitabine in patients with biliary cancer in a phase II study that demonstrated sufficient antitumor activity of weekly Genexol-PM without severe adverse events (ORR, 25.6%; disease control rate, 71.8%) [27]. Accordingly, we chose 100 mg/m2 of weekly Genexol-PM as the starting dose for this trial.
In conclusion, based on our findings in which no DLTs were found and no MTD was detected at the tested doses of Genexol-PM, we suggest that the RP2D of weekly Genexol-PM can be set as 120 mg/m2 when combined with carboplatin. Further studies on weekly Genexol-PM are needed to evaluate its efficacy, safety, and long-term outcomes in patients with gynecologic cancer.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study protocol was approved by the institutional review board of Asan Medical Center (approval date: November 26, 2015; approval #2015-1296) and adhered to the principles in the Declaration of Helsinki. All participants provided written informed consent. The study was performed in compliance with the Declaration of Helsinki and was registered in ClinicalTrials.gov (NCT02739529).
Acknowledgments
We thank all patients and their families who participated in this phase I study. We also thank the study-site staff who assisted with coordination and medical monitoring. Finally, we thank Dr. Joon Seo Lim from the Scientific Publications Team at Asan Medical Center for his assistance in the preparation of the manuscript.
References
1. Ovarian cancer (version 1.2022) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2022 [cited 2022 Jan 18]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
.
2. Uterine neoplasms (version 1.2022) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2022 [cited 2021 Nov 4]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf
.
3. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003; 21:3194–200.

4. du Bois A, Neijt JP, Thigpen JT. First line chemotherapy with carboplatin plus paclitaxel in advanced ovarian cancer: a new standard of care? Ann Oncol. 1999; 10(Suppl 1):35–41.
5. Miller DS, Filiaci VL, Mannel RS, Cohn DE, Matsumoto T, Tewari KS, et al. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J Clin Oncol. 2020; 38:3841–50.

6. Banerjee S, Moore KN, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021; 22:1721–31.

7. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020; 38:1–10.

8. Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018; 81:17–38.

9. Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009; 374:1331–8.

10. Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013; 14:1020–6.

11. Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014; 15:396–405.

12. Mathew AE, Mejillano MR, Nath JP, Himes RH, Stella VJ. Synthesis and evaluation of some water-soluble prodrugs and derivatives of taxol with antitumor activity. J Med Chem. 1992; 35:145–51.

13. Lee SW, Kim YM, Kim YT, Kang SB. An open-label, multicenter, phase I trial of a cremophor-free, polymeric micelle formulation of paclitaxel combined with carboplatin as a first-line treatment for advanced ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG-3016). J Gynecol Oncol. 2017; 28:e26.

14. Kloover JS, den Bakker MA, Gelderblom H, van Meerbeeck JP. Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Cancer. 2004; 90:304–5.

15. Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Wan Kim S, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001; 72:191–202.

16. Lee SW, Kim YM, Cho CH, Kim YT, Kim SM, Hur SY, et al. An open-label, randomized, parallel, phase II trial to evaluate the efficacy and safety of a cremophor-free polymeric micelle formulation of paclitaxel as first-line treatment for ovarian cancer: a Korean Gynecologic Oncology Group Study (KGOG-3021). Cancer Res Treat. 2018; 50:195–203.

17. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.
18. Rustin GJ. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003; 21:187s–93s.

19. Clamp AR, James EC, McNeish IA, Dean A, Kim JW, O’Donnell DM, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet. 2019; 394:2084–95.

20. Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ, Walker JL, et al. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016; 374:738–48.

21. Ba Y, Shi Y, Jiang W, Feng J, Cheng Y, Xiao L, et al. Current management of chemotherapy-induced neutropenia in adults: key points and new challenges: Committee of Neoplastic Supportive-Care (CONS), China Anti-Cancer Association Committee of Clinical Chemotherapy, China Anti-Cancer Association. Cancer Biol Med. 2020; 17:896–909.

22. Scripture CD, Figg WD, Sparreboom A. Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol. 2006; 4:165–72.

23. Boulanger J, Boursiquot JN, Cournoyer G, Lemieux J, Masse MS, Almanric K, et al. Management of hypersensitivity to platinum- and taxane-based chemotherapy: cepo review and clinical recommendations. Curr Oncol. 2014; 21:e630–41.

24. Shawky H, Tawfik H, Hewidy M. Weekly dose-dense paclitaxel and carboplatin in recurrent ovarian carcinoma: a phase II trial. J Egypt Natl Canc Inst. 2014; 26:139–45.

25. Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther. 2015; 9:3767–77.

26. Lim WT, Tan EH, Toh CK, Hee SW, Leong SS, Ang PC, et al. Phase I pharmacokinetic study of a weekly liposomal paclitaxel formulation (Genexol-PM) in patients with solid tumors. Ann Oncol. 2010; 21:382–8.

27. Kim JY, Do YR, Song HS, Cho YY, Ryoo HM, Bae SH, et al. Multicenter phase II clinical trial of Genexol-PM(R) with gemcitabine in advanced biliary tract cancer. Anticancer Res. 2017; 37:1467–73.
Fig. 1
Study design during dose escalation steps. AUC, area under the curve; DLT, dose-limiting toxicity; MTD, maximum tolerated dose.
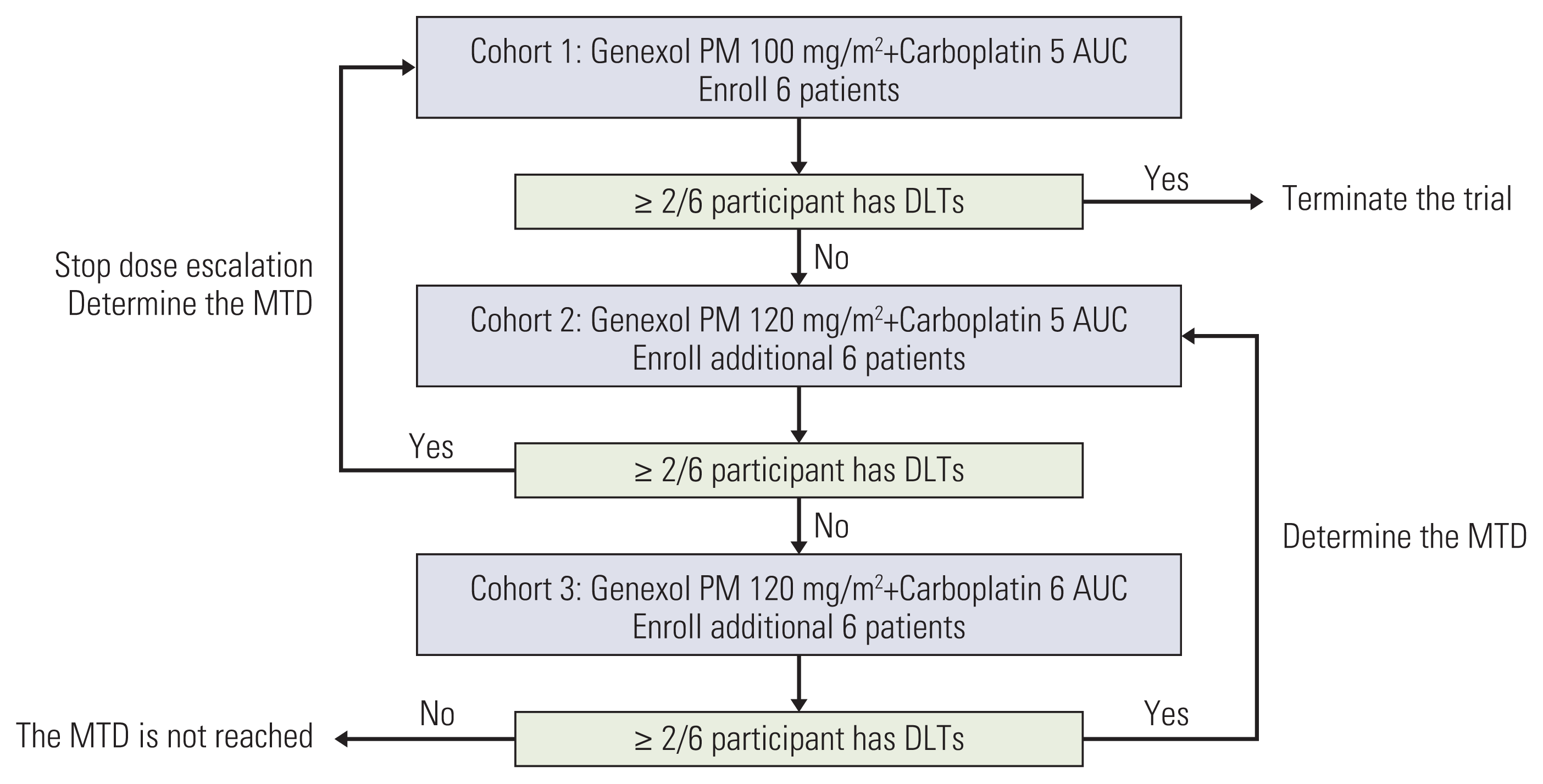
Table 1
Characteristics of the study patients
Table 2
Dosage escalation scheme and administration of treatments
Table 3
Adverse drug reactions per patient stratified by dose level
Table 4
Serious adverse events
| Serious adverse events |
Genexol PM 100 mg/m2+ Carboplatin 5 AUC |
Genexol PM 120 mg/m2+ Carboplatin 5 AUC |
Genexol PM 120 mg/m2+ Carboplatin 6 AUC |
|||
|---|---|---|---|---|---|---|
| No. of patients | No. of casesa) | No. of patients | No. of casesa) | No. of patients | No. of casesa) | |
| Hematologic disorders | ||||||
| Neutropenia | 1 | 1 | 1 | 2 | 0 | 0 |
| Infections | ||||||
| Pneumonia | 0 | 0 | 1 | 1 | 0 | 0 |
| Total | 1 | 1 | 1 | 3 | 0 | 0 |
Table 5
Treatment response rate stratified by dose level




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download