Abstract
Posttransplant lymphoproliferative disorders (PTLDs) are severe complications with heterogeneous clinical pictures involving abnormal lymphoproliferation in solid organ transplants and are known to be closely associated with Epstein-Barr virus (EBV) infection. Herein, we present a case of graft lymphoma in a febrile kidney transplant recipient. A 37-year-old woman was admitted with an abrupt 39 °C fever, mild graft discomfort, and gross hematuria. She had received deceased donor kidney transplantation 8 years earlier, but developed graft failure due to a recurrence of immunoglobulin A nephropathy. Laboratory tests revealed anemia and elevated levels of inflammatory markers. Enhanced abdominopelvic computed tomography showed graft swelling with perirenal fat stranding. Thus, we administered antibiotics for a urinary tract infection and increased the doses of steroids due to suspicion of graft intolerance syndrome. However, the patient's symptoms gradually worsened. Eventually, we performed graft nephrectomy and histologically confirmed EBV-positive diffuse large B cell lymphoma. We report a case in which a PTLD was considered in the differential diagnosis of a kidney transplant recipient with symptoms similar to those of a urinary tract infection or graft intolerance syndrome.
Posttransplant lymphoproliferative disorders (PTLDs) are significant complications in recipients of solid organ transplants, as they involve various lymphoid and/or plasmacytic proliferations. These disorders occur in 1% to 3% of kidney transplant recipients, who face a lifetime risk that is eight times higher than that of the general population [1,2]. The Epstein-Barr virus (EBV) infection is the most probable cause of PTLDs. However, diminished T cell-mediated immune surveillance and other viral infections, such as hepatitis C and cytomegalovirus (CMV), may also contribute to their development.
The clinical manifestation of PTLDs can range from completely asymptomatic forms to severe varieties that are accompanied by organ failure [3]. In these instances, it becomes crucial to distinguish PTLDs from infections or instances of graft rejection.
Here, we present a case involving a kidney transplant recipient who developed a pathologically confirmed lymphoma following a nephrectomy. This patient exhibited symptoms of allograft dysfunction, with a clinical presentation suggestive of urinary tract infection (UTI) and graft intolerance syndrome.
This study was conducted in compliance with the principles of the Declaration of Helsinki. This study was approved by the Institutional Review Board of Jeonbuk National University Hospital (IRB No. CUH 2023-06-014). The requirement for informed consent was waived because this study was conducted through a retrospective review of medical records.
In September 2020, a 37-year-old woman was admitted due to a sudden onset of a 39 °C fever. She had received a kidney transplant from a deceased donor at the age of 29, as a treatment for end-stage renal disease caused by immunoglobulin A (IgA) nephropathy. Three years posttransplant, we conducted a graft biopsy due to a rise in her serum creatinine levels, which confirmed a recurrence of IgA nephropathy. Ultimately, the graft failed, leading her to undergo continuous ambulatory peritoneal dialysis in April 2020. At the time of her transplant, she was given basiliximab induction therapy, and her ongoing immunosuppressive regimen included tacrolimus, mycophenolate mofetil, and prednisolone. Following the failure of the transplant, we gradually reduced the dosage of tacrolimus by 25% each week, stabilizing it at 0.25 mg twice daily. We also tapered the doses of mycophenolate and prednisolone.
Two months later, the patient reported experiencing intermittent gross hematuria during an outpatient department visit. Concurrently, her hemoglobin level had decreased to 6.3 g/dL from a baseline of 9.7 g/dL, indicating a worsening of anemia that was resistant to treatment with an erythropoiesis-stimulating agent. We attempted to manage her symptoms by increasing the dosage of the immunosuppressive agent, which led to a temporary improvement. However, her symptoms deteriorated once we began to taper the dosage. In September 2020, she began to exhibit additional symptoms, including fever and mild discomfort in the graft area, which necessitated her hospitalization.
On admission, her complete blood count revealed a white blood cell count of 4.9 × 103/μL and a hemoglobin level of 5.5 g/dL. Her chemistry profile was as follows: total protein, 5.0 g/dL; albumin, 3.0 g/dL; blood urea nitrogen, 54 mg/dL; creatinine, 8.5 mg/dL; lactate dehydrogenase, 1,453 IU/L; high-sensitivity C-reactive protein, 187 mg/L; transferrin saturation, 31%; and ferritin, 1,080 ng/mL. A peripheral blood smear indicated normochromic normocytic anemia accompanied by poikilocytosis. Both urine and blood cultures were negative. Serologic tests for EBV showed negative results for viral capsid antigen (VCA) immunoglobulin M (IgM) and positive results for VCA immunoglobulin G (IgG). A physical examination revealed tenderness in the graft area, but there were no palpable lymph nodes, hepatosplenomegaly, or other masses. Enhanced computed tomography displayed graft swelling and multiple low-attenuated areas with calyceal dilatation in the transplanted kidney (Fig. 1). We promptly initiated antibiotic treatment. However, the patient's fever did not subside, and her condition weakened compared to her prehospitalization state. Two days later, due to the uncontrolled graft intolerance syndrome and graft abscess, we performed graft nephrectomy. Following the procedure, the patient's symptoms and inflammatory marker levels improved. She continued on a regimen of prednisolone 5 mg daily, which was discontinued after a month.
The histological analysis revealed the diffuse infiltration of atypical lymphoid cells between the sclerotic glomerulus and atrophic renal tubules. The patient also tested positive for CD20, Ki67, and EBV-encoded small nuclear RNAs via in situ hybridization (Fig. 2), indicating EBV-positive diffuse large B cell lymphoma. The patient did not exhibit MYC or BCL2 rearrangements, and the serum EBV polymerase chain reaction was positive with 1.3×104 copies/mL. Subsequently, she was referred to an oncologist and underwent a staging workup, which included positron emission tomography/computed tomography and a bone marrow examination. She was diagnosed with stage 1 disease without nodal involvement and underwent four cycles of a treatment regimen that included cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine, resulting in complete remission.
The long-term use of immunosuppressants to prevent allograft rejection in solid organ transplant patients can lead to an increased incidence of de novo malignancy, which in turn, contributes to mortality. In a population-based nationwide study conducted in South Korea, it was found that approximately 7% of all kidney transplant recipients developed cancer. The incidence of PTLDs, including Kaposi's sarcoma and non-Hodgkin lymphoma, was higher than in the general population when considering the standardized incidence ratio [4].
PTLDs are classified into early-onset and late-onset types, based on their time of manifestation posttransplantation. Each type exhibits distinct characteristics. The early-onset type typically appears within the first year following transplantation and is associated with EBV infection, allograft involvement, polymorphic type, and a more favorable response to reduced immunosuppressive agents. Conversely, the late-onset type is characterized by a lack of EBV, extranodal involvement, and monomorphic type in older patients. This type often necessitates more aggressive chemotherapy [5]. In this particular case, malignancy was confirmed 8 years posttransplantation, suggesting a closer alignment with the early-onset type, despite being an EBV-associated lymphoma. Furthermore, the detection of lymphoma solely within the graft, without invasion of other organs, sets this case apart from others.
The factors contributing to anemia following graft failure encompass diminished renal erythropoietin production, impaired iron metabolism, inflammation, the presence of uremic toxins, vitamin deficiencies, secondary hyperparathyroidism, and a decrease in red cell longevity [6]. Given this patient's initial symptoms and persistent anemia, the differential diagnosis considered both infection and graft intolerance syndrome. As a result, we administered antibiotics and increased steroid treatment. Despite these interventions, the patient's condition did not improve, leading us to perform nephrectomy. The diagnosis was lymphoma, which was subsequently treated appropriately.
In summary, graft lymphoma manifested as persistent severe anemia and an abruptly high fever. Thus, even if transplant recipients return to dialysis after graft failure, PTLDs should be included in the differential diagnosis with a high index of suspicion in addition to UTI and graft intolerance syndrome in patients with symptoms such as fever, gross hematuria, and graft tenderness.
ARTICLE INFORMATION
REFERENCES
1. Opelz G, Döhler B. 2004; Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 4:222–30. DOI: 10.1046/j.1600-6143.2003.00325.x. PMID: 14974943.
2. Francis A, Johnson DW, Teixeira-Pinto A, Craig JC, Wong G. 2018; Incidence and predictors of post-transplant lymphoproliferative disease after kidney transplantation during adulthood and childhood: a registry study. Nephrol Dial Transplant. 33:881–9. DOI: 10.1093/ndt/gfx356. PMID: 29342279.
3. Park H, Kim JS, Kong JH, Kim SH, Park SW, Song SH, et al. 2016; Post-transplant lymphoproliferative disorder in transplanted kidney causing urinary tract obstruction. J Korean Soc Transplant. 30:44–9. DOI: 10.4285/jkstn.2016.30.1.44.
4. Kim B, Kang M, Kim Y, Lee HS, Kim B, Lee JJ, et al. 2021; De novo cancer incidence after kidney transplantation in South Korea from 2002 to 2017. J Clin Med. 10:3530. DOI: 10.3390/jcm10163530. PMID: 34441826. PMCID: PMC8396914.
5. San-Juan R, Comoli P, Caillard S, Moulin B, Hirsch HH, Meylan P. 2014; Epstein-Barr virus-related post-transplant lymphoproliferative disorder in solid organ transplant recipients. Clin Microbiol Infect. 20 Suppl 7:109–18. DOI: 10.1111/1469-0691.12534. PMID: 24475976.
6. Yabu JM, Winkelmayer WC. 2011; Posttransplantation anemia: mechanisms and management. Clin J Am Soc Nephrol. 6:1794–801. DOI: 10.2215/CJN.01190211. PMID: 21734096.
Fig. 1
(A) Enhanced computed tomography showed graft swelling and multiple low-attenuated areas with calyceal dilatation in the transplanted kidney. Internal calcification and peripheral fat stranding were also confirmed. (B) A similar finding was observed in the coronary section. (C) A low-attenuating infiltrative lesion was found around the ureter of the transplanted kidney, but there was no hydronephrosis.
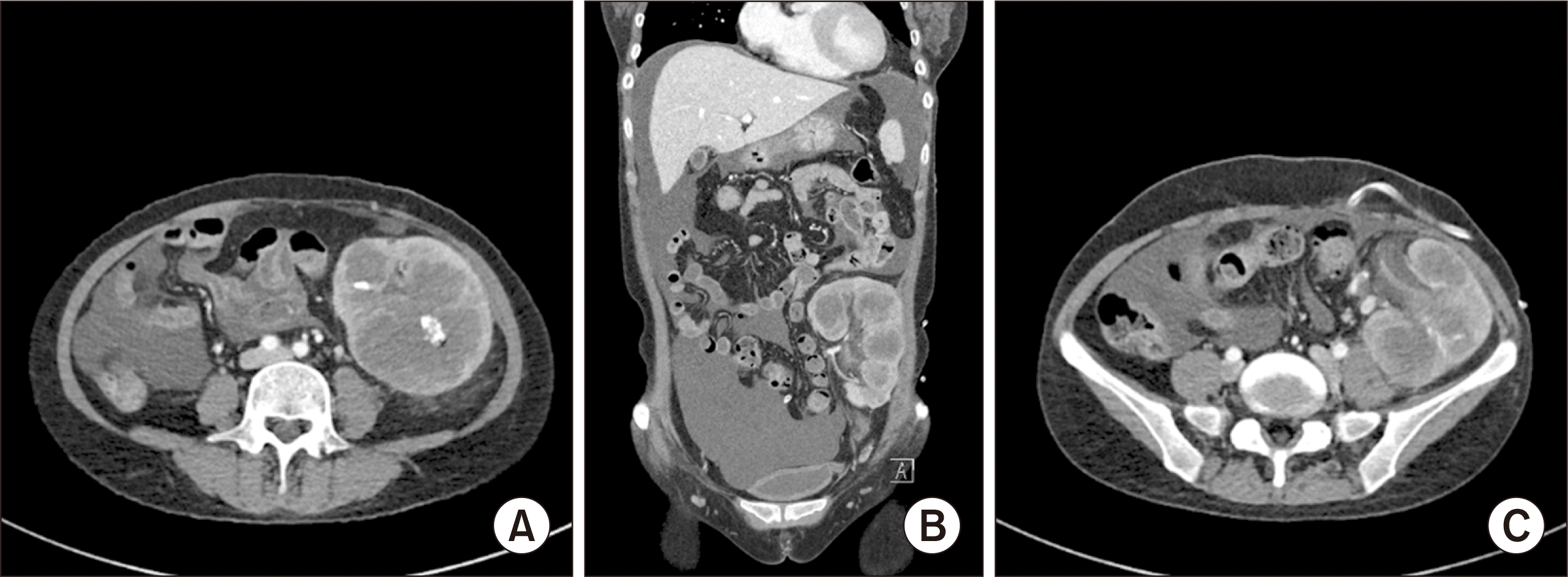
Fig. 2
(A) Atypical lymphoid cells infiltrate diffusely between sclerotic glomerulus (asterisk) and atrophic renal tubules (arrows). (B) The tumor cells have large nuclei with conspicuous nucleoli. (C) The tumor cells are positive for CD20 (D) but negative for CD3. (E) The tumor cells are positive for EBER in situ hybridization and (F) show a high Ki67-positive proliferation index. Original magnification: (A) ×100, (B-F) ×400. (A, B) Hematoxyline and eosin stain, (C–F) Immunohistochemical stain.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download