Abstract
Performing kidney transplantations in patients with morbid obesity presents unique challenges using the conventional retroperitoneal approach. Robot-assisted kidney transplantation (RAKT) offers several advantages, such as better access to hard-to-reach areas. A 56-year-old morbidly obese woman presented with end-stage renal disease due to diabetic nephropathy. The patient had a history of obesity for over 20 years, with a peak body mass index (BMI) of 46.9 kg/m2. Before transplantation, she successfully reduced her BMI to 28.9 kg/m2, but was left with excessive skin folds. The surgery began with the removal of the sac from the incisional hernia and umbilical hernia, which was then used as the site for the GelPOINT port. The da Vinci X robot system was utilized to perform RAKT. After completing RAKT, the plastic surgery team initiated abdominal reconstruction involving panniculectomy, followed by hernial reconstruction and abdominoplasty. The patient’s postoperative course was uneventful, and she was discharged on postoperative day 7. Her creatinine level was 0.69 mg/dL, and she did not experience any episodes of rejection during the 16 months following RAKT. This case report describes the successful combination of RAKT with incisional hernia reconstruction and abdominoplasty in a patient with morbid obesity.
Go to : 
Go to : 
In recent years, obesity has become a major global health concern [1] and has grown increasingly prevalent among patients with end-stage renal disease (ESRD) [2,3]. For non-overweight and obese patients alike, kidney transplantation (KT) is the only solution for individuals with progressing renal disease to cease reliance on dialysis. However, obesity presents additional challenges for individuals with ESRD, such as higher risks for graft failure and postoperative complications following KT. Moreover, morbid obesity is associated with numerous coexisting medical conditions, including insulin resistance, diabetes mellitus, and elevated intra-abdominal pressure due to increased abdominal fat, which can lead to gastroesophageal reflux, stress urinary incontinence, venous stasis disease, and abdominal hernia requiring medical attention [4].
The concept of using robotic surgery for KT emerged from the need to minimize the complications associated with open KT. Since a team at the University of Illinois at Chicago completed the first-ever intra-abdominal robot-assisted kidney transplant (RAKT) on an overweight patient in 2009, RAKT has been considered a viable alternative in obese individuals [5] due to several benefits, including enhanced precision with advanced instruments, better visualization of the surgical field, and the availability of minimally invasive techniques with smaller incisions and less tissue damage. These benefits reduce morbidity and result in faster recovery, which is particularly important for obese patients who may already have underlying health conditions that put them at risk for complications.
Open surgery can also be challenging for obese patients with coexisting medical conditions such as hernias. Nonetheless, performing hernia repair simultaneously with KT can be advantageous, as both procedures can be done under a single administration of general anesthesia. Obese patients who have lost significant amounts of weight before KT may have excess skin, known as a panniculus, which should be removed to improve the cosmetic appearance and reduce complications such as panniculitis or other skin infections. This case report presents a successful combination of RAKT, incisional hernia reconstruction, and abdominoplasty in a morbidly obese patient with multiple comorbidities.
Go to : 
This study was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2022-0909), which waived the requirement for informed consent due to the retrospective nature of the study.
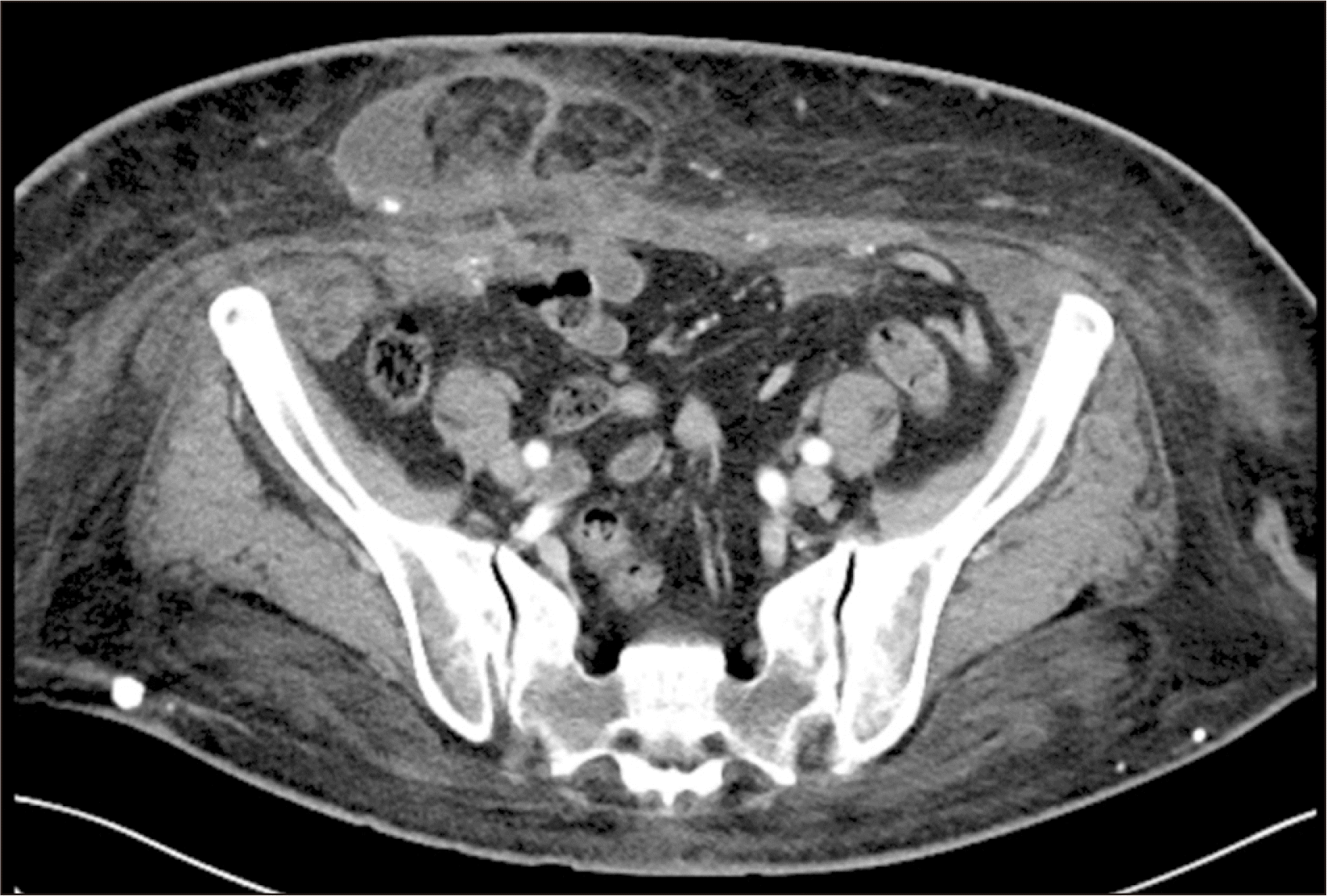
A 56-year-old morbidly obese woman with a body mass index (BMI) of 33.2 kg/m2 presented with ESRD due to diabetic nephropathy. The patient had a history of obesity for over 20 years, with a peak BMI of 46.9 kg/m2. Prior to surgery, the patient was advised to follow a strict diet and participate in regular physical activity to achieve weight loss. As a result, she managed to lower her BMI to 28.9 kg/m2; however, she was left with surplus skin folds and loose skin that caused physical discomfort and affected her external appearance. In addition, she had visible bulging and a 4.3-cm herniation of abdominal contents through the surgical incision from an earlier hysterectomy (Fig. 1). She had multiple comorbidities, including hypertension, dyslipidemia, diabetes mellitus, and hypothyroidism; she also had a history of transient ischemic attack, for which she was receiving aspirin. The patient was evaluated for KT and was deemed a suitable candidate for RAKT.
Robot-assisted kidney transplant
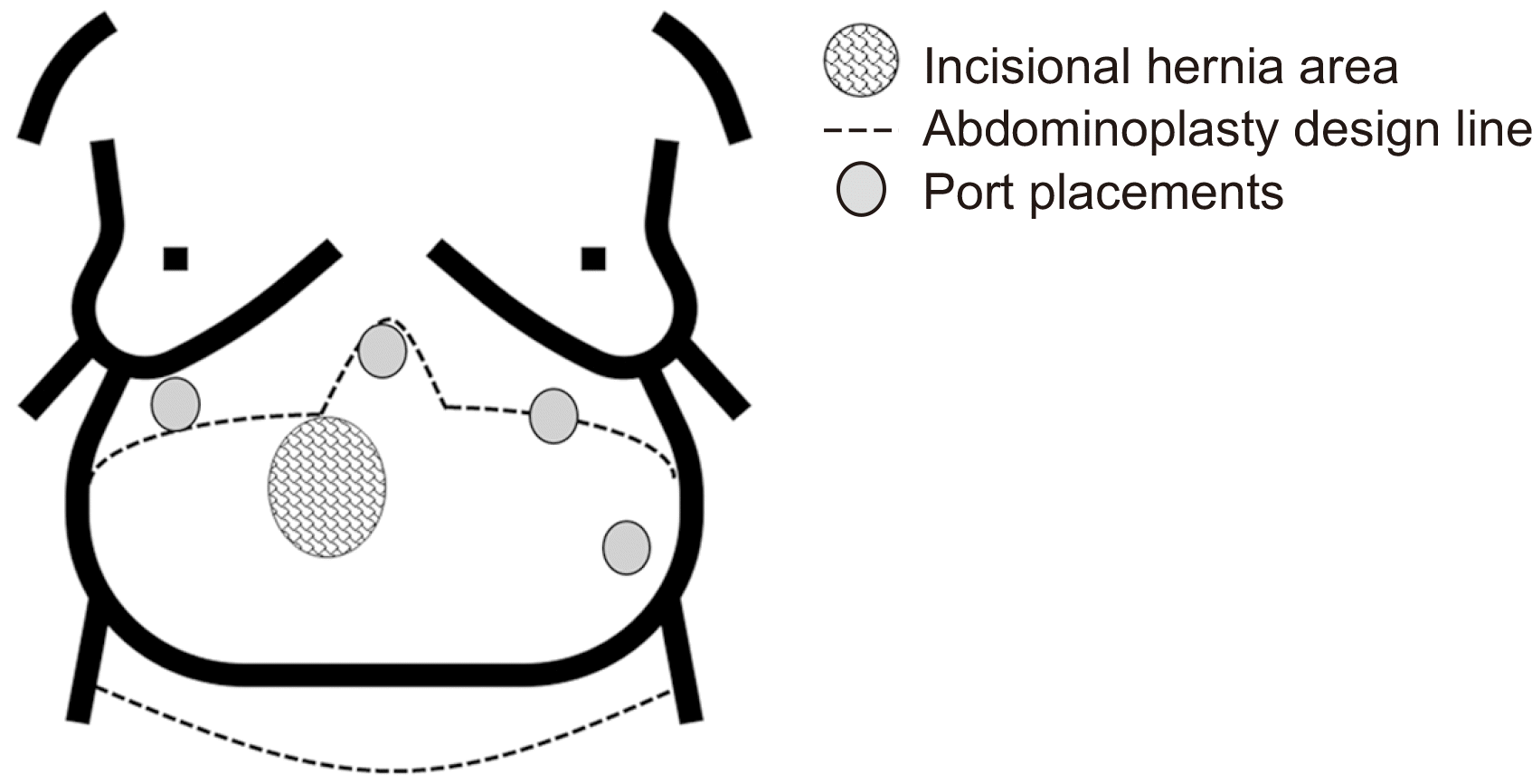
During the surgical operation for RAKT, the patient was placed at a 45° angle in the Trendelenburg position and secured to the operating table using straps. Her arms were kept close to her body, and gelfoam pads were utilized to prevent pressure points and excessive compression. The anesthetic team ensured that the patient’s breathing was not obstructed. The positioning of the ports during surgery is shown in Fig. 2. Instead of the standard large right lower quadrant incision commonly utilized for the entry port for kidney graft introduction and a GelPOINT port (Applied Medical), our team made a horizontal incision over the incisional hernia. Once the sac was visible, the hernia was reduced, and the hernia sac was dissected from the surrounding tissues. This incision was then utilized as the insertion point for the GelPOINT port.
The RAKT procedure was conducted with the aid of the da Vinci Xi Robot (Intuitive Surgical). The robot had four arms and a 0° lens. Once the iliac vessels were exposed and an extraperitoneal pouch was created, the bladder was separated to prepare for the ureteroneocystostomy procedure. The graft, which was enclosed in a gauze jacket, was introduced into the abdominal cavity through the GelPOINT device. To induce regional hypothermia, 200 to 250 mL of ice slush was used to cool the graft via the GelPOINT.
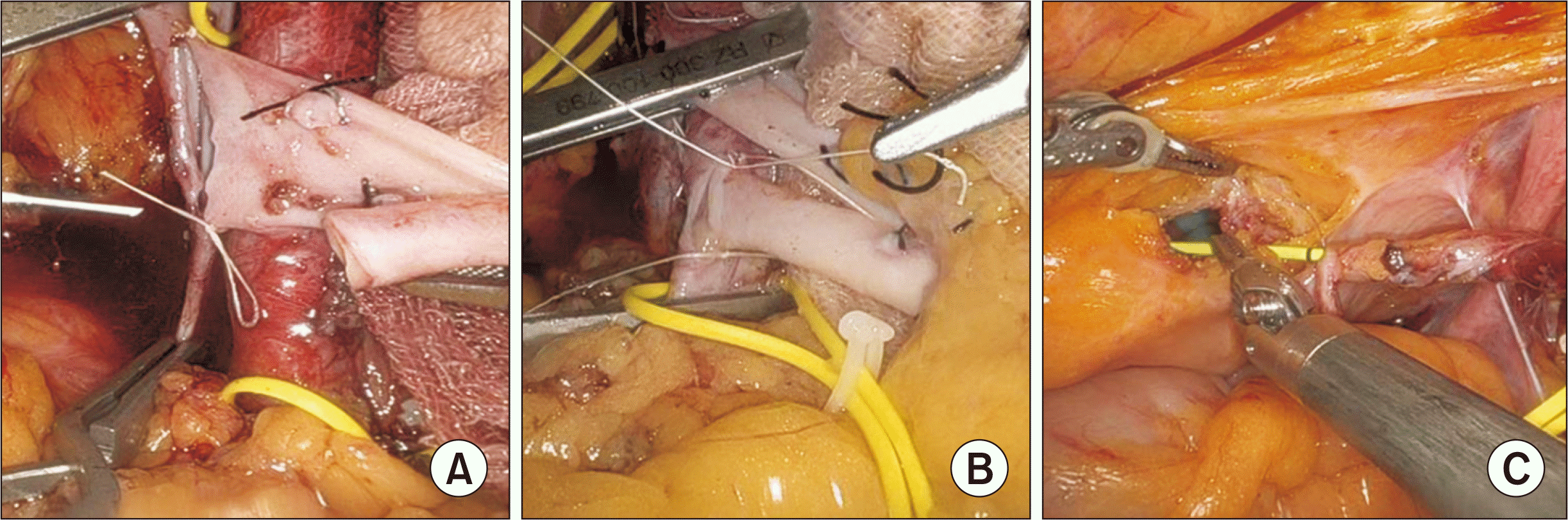
After a venotomy was made with curved cold scissors, the renal vein of the graft was joined to the external iliac vein in a continuous end-to-side manner (Fig. 3A). Additional ice slush was added after the venous anastomosis to keep the graft cool. Next, the external iliac artery was clamped, and robotic curved cold scissors were used to create a linear arteriotomy, which was later transformed into a circular shape with an aortic punch device and washed with heparinized saline. The renal artery was then connected to the external iliac artery in a continuous end-to-side manner (Fig. 3B). After the anastomosis was confirmed as secure, blood supply to the kidney was re-established and examined for color and turgor. During the entire anastomosis procedure, the air pressure was lowered to 8 mmHg to detect bleeding.
Afterward, the kidney was moved into the extraperitoneal pouch, and the ureterovesical anastomosis was performed using a modified Lich-Gregoir technique. Two semi-continuous sutures were made with 4-0 Monocryl sutures (Ethicon Inc.) over the previously inserted 6F, 14-cm double-J stent (Fig. 3C). The detrusor muscle was then closed using a continuous suture to form an anti-reflux mechanism.
Incisional hernia mesh repair and abdominoplasty
Following the completion of RAKT, the patient’s position was changed from Trendelenburg to supine, and the plastic surgery team commenced abdominal reconstruction. This involved performing an abdominal panniculectomy to remove surplus skin and subcutaneous tissue, followed by hernia reconstruction using a mesh and abdominoplasty.
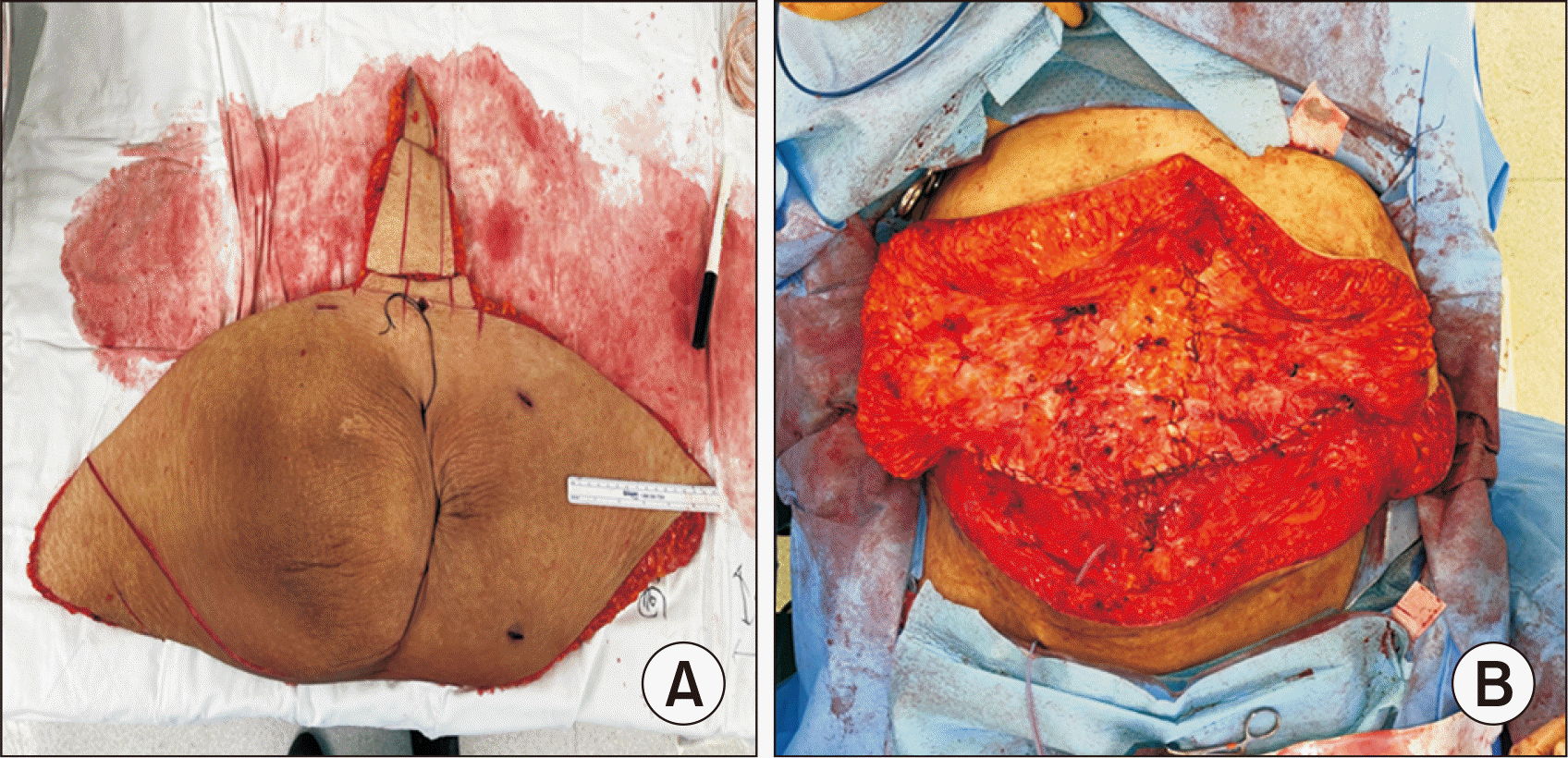
To carry out the abdominoplasty procedure, an incision was created above the pubic bone and extended to the iliac crest (Fig. 4). The adipo-cutaneous flap was separated from the underlying tissues and elevated to the xiphoid-costal arch while preserving the umbilicus and its stalk. The excess skin tissue was then excised, and a new location was fashioned for the umbilicus. Lastly, drainage tubes were placed, and the different layers of tissue were sutured together.
The transplantation and reconstruction were performed under general anesthesia and completed successfully in a total operating time of 490 minutes. RAKT itself took 285 minutes, with 173 minutes of cold ischemia time, 4 minutes of warm ischemia time, 46 minutes of anastomosis time, and 69 minutes of rewarming time. Once reperfusion began, the kidney started functioning immediately, and the patient’s recovery after the operation was uneventful. The patient began moving around and eating normally shortly after the surgery. She experienced only slight pain, which quickly subsided during the postoperative period; the patient noted that this pain was less severe than the pain she had experienced after her earlier hysterectomy. The immunosuppression regimen consisted of tacrolimus, mycophenolate mofetil, and prednisone. The postoperative course was uneventful, and she was discharged on postoperative day 7.
The patient’s concerns regarding both aesthetic and functional aspects were appropriately addressed; at a 6-month follow-up appointment, she expressed that the procedure had not only met but exceeded her expectations in terms of both functional enhancement and cosmetic appearance. At 6 months posttransplantation, the patient’s creatinine level was stable at 0.69 mg/dL, and the patient reported improved quality of life. The patient has continued to follow up regularly with the transplant team, and there have been no complications or rejection episodes as of the writing of this report.
Go to : 
Morbid obesity in KT patients is associated with various negative outcomes, including delayed graft function, wound complications, prolonged hospital stays, new diagnoses of diabetes mellitus, biopsy-confirmed acute rejection, and graft failure [6-9]. Specifically, excess skin in obese individuals may hinder their ability to carry out normal activities and exercise, which could potentially hinder the recovery process following KT; therefore, removing excess skin can improve the patient’s aesthetic appearance and increase their satisfaction with the surgery, motivating them to maintain a healthy diet and lifestyle. Previous studies reported that patients with a large pannus are at a higher risk of developing panniculitis, which can lead to kidney failure [10,11]. This is important because KT recipients are administered a high dose of induction immunosuppression, which increases the risk of infection. One possible strategy to reduce the incidence of wound complications in obese patients with large skin flaps involves panniculectomy or abdominoplasty procedures to remove excess skin. However, since these procedures require additional general anesthesia, it is better to perform panniculectomy or abdominoplasty during the transplantation surgery to avoid the need for staged surgery, which results in a longer recovery and wound healing time.
Ngaage et al. [12] reported on the results of an 8-year experience of performing panniculectomy during living donor renal transplantation; according to their results, performing concomitant panniculectomy with KT did not increase the risk of mortality or complications in the process of postsurgical management. Furthermore, there is a significant body of literature that demonstrates the safety of combining abdominoplasty with other abdominal surgical procedures, such as hernia repair, without increasing the risk of wound complications [13,14].
A systematic review and meta-analysis reported that the surgery time for living donor renal transplantation in obese patients is longer than that in the general population, with an estimated average duration of 314 minutes (range, 216–390 minutes) for patients with a BMI greater than 30 kg/m2 [15]. Despite the fact that RAKT generally takes longer than open surgery, it may take less time or the same amount of time in morbidly obese patients due to easier access and greater flexibility, as demonstrated in our current case.
The patient’s previous success in weight reduction before KT was overshadowed by the challenge posed by excessive skin flaps resulting from the weight loss. Conducting an open KT procedure in this case would have been further complicated by the presence of abundant adipose tissue, including visceral fat, which would have obstructed the surgical field of view. It is important to note that abdominoplasty does not involve actual abdominal incisions. Introducing an open wound for KT in this patient would undoubtedly have prolonged the hospitalization. In contrast, opting for minimally invasive RAKT in the initial stage of surgery would have had a significant positive impact on the duration of hospital stay, despite the eventual occurrence of a sizable wound from the abdominoplasty.
Typically, patients who undergo abdominoplasty at our institution are hospitalized for around 2 days, while those who have incisional hernia repair stay for 2 or 3 days and those who undergo RAKT alone usually stay for 5 days. The patient described in this report had a combination of all three procedures and was discharged on the seventh day after the operation. This shows that performing hernia repair, abdominoplasty, and RAKT together is a viable surgical method that may not necessarily increase the hospitalization period.
A primary objective of the robotic procedure was to minimize surgical trauma and promote a quicker recovery by using small incisions. To achieve this, we substituted the typical large incision in the right lower quadrant for the incision used in incisional hernia sac repair in order to reduce the risk of postoperative complications such as wound infections.
To the best of our knowledge, this is the first case of RAKT combined with an incisional hernia repair followed by abdominoplasty in a morbidly obese patient. In this case, RAKT offered several benefits, including the ability to enhance precision and technical skill in vascular anastomoses, similar to open surgery. Furthermore, RAKT reduced surgical morbidity, which is critical for vulnerable and immunocompromised patients with ESRD and obesity.
In summary, our case demonstrates the safety of performing RAKT in a morbidly obese patient with well-coordinated surgical plans involving plastic surgeons to combine hernia repair and abdominoplasty. Therefore, robotic KT with combined hernia repair and abdominoplasty may be considered as a potential option in morbidly obese patients who are at risk of complications due to their obesity, as well as those who may be prone to panniculitis after transplantation due to a large panniculus from intensive body weight reduction or to incisional hernias from previous abdominal surgery.
Go to : 
ARTICLE INFORMATION
Author Contributions
Conceptualization: JMK, SS. Data curation: JMK, SS. Formal analysis: JMK, SS. Funding acquisition: SS. Visualization: HEK, HK, EKK. Writing–original draft: JMK, SS. Writing–review & editing: YK, JHJ, YHK, SS. All authors read and approved the final manuscript.
Go to : 
REFERENCES
1. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. GBD 2015 Obesity Collaborators. 2017; Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 377:13–27. DOI: 10.1056/NEJMoa1614362. PMID: 28604169. PMCID: PMC5477817.
2. Ladhani M, Craig JC, Irving M, Clayton PA, Wong G. 2017; Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 32:439–49. DOI: 10.1093/ndt/gfw075. PMID: 27190330.
3. Whaley-Connell A, Sowers JR. 2017; Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 92:313–23. DOI: 10.1016/j.kint.2016.12.034. PMID: 28341271.
4. Lambert DM, Marceau S, Forse RA. 2005; Intra-abdominal pressure in the morbidly obese. Obes Surg. 15:1225–32. DOI: 10.1381/096089205774512546. PMID: 16259876.
5. Giulianotti P, Gorodner V, Sbrana F, Tzvetanov I, Jeon H, Bianco F, et al. 2010; Robotic transabdominal kidney transplantation in a morbidly obese patient. Am J Transplant. 10:1478–82. DOI: 10.1111/j.1600-6143.2010.03116.x. PMID: 20486912.
6. Modlin CS, Flechner SM, Goormastic M, Goldfarb DA, Papajcik D, Mastroianni B, et al. 1997; Should obese patients lose weight before receiving a kidney transplant? Transplantation. 64:599–604. DOI: 10.1097/00007890-199708270-00009. PMID: 9293872.
7. Orofino L, Pascual J, Quereda C, Burgos J, Marcen R, Ortuño J. 1997; Influence of overweight on survival of kidney transplant. Nephrol Dial Transplant. 12:855. DOI: 10.1093/ndt/12.4.855. PMID: 9141040.
8. Beddhu S, Pappas LM, Ramkumar N, Samore M. 2003; Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 14:2366–72. DOI: 10.1097/01.ASN.0000083905.72794.E6. PMID: 12937315.
9. Holley JL, Shapiro R, Lopatin WB, Tzakis AG, Hakala TR, Starzl TE. 1990; Obesity as a risk factor following cadaveric renal transplantation. Transplantation. 49:387–9. DOI: 10.1097/00007890-199002000-00032. PMID: 2305469. PMCID: PMC2965531.
10. Richens G, Piepkorn MW, Krueger GG. 1982; Calcifying panniculitis associated with renal failure: a case of Selye's calciphylaxis in man. J Am Acad Dermatol. 6(4 Pt 1):537–9. DOI: 10.1016/S0190-9622(82)70046-5. PMID: 7076908.
11. Lugo-Somolinos A, Sánchez JL, Méndez-Coll J, Joglar F. 1990; Calcifying panniculitis associated with polycystic kidney disease and chronic renal failure. J Am Acad Dermatol. 22(5 Pt 1):743–7. DOI: 10.1016/0190-9622(90)70101-M. PMID: 2347961.
12. Ngaage LM, Elegbede A, Tadisina KK, Gebran SG, Masters BM, Rada EM, et al. 2019; Panniculectomy at the time of living donor renal transplantation: an 8-year experience. Am J Transplant. 19:2284–93. DOI: 10.1111/ajt.15285. PMID: 30720924.
13. Zemlyak AY, Colavita PD, El Djouzi S, Walters AL, Hammond L, Hammond B, et al. 2012; Comparative study of wound complications: isolated panniculectomy versus panniculectomy combined with ventral hernia repair. J Surg Res. 177:387–91. DOI: 10.1016/j.jss.2012.06.029. PMID: 22795269.
14. Berry MF, Paisley S, Low DW, Rosato EF. 2007; Repair of large complex recurrent incisional hernias with retromuscular mesh and panniculectomy. Am J Surg. 194:199–204. DOI: 10.1016/j.amjsurg.2006.10.031. PMID: 17618804.
15. Lafranca JA, IJermans JN, Betjes MG, Dor FJ. 2015; Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med. 13:111. DOI: 10.1186/s12916-015-0340-5. PMID: 25963131. PMCID: PMC4427990.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download