Abstract
Background
Accurately predicting the demand for blood transfusions is crucial for blood banks. Given the potential for emergency situations, it is imperative that blood banks maintain a sufficient inventory of blood products. In this study, we examined the use of perioperative transfusions in patients undergoing elective kidney transplants.
Methods
Data on all complement-dependent cytotoxicity-crossmatched assays between 2013 and 2022 were collected. We excluded repeated assays and patients who did not undergo kidney transplantation. Transfusion records and transfusion adverse reactions were reviewed retrospectively.
Results
In total, 30 patients underwent elective kidney transplantation from 2013 to 2022. The mean age of the patients was 48.1±9.7 years. The male-to-female ratio was 1.51. Four patients received transfusions intraoperatively, whereas eight patients were transfused postoperatively. The postoperative hemoglobin level of the transfusion group (n=9, 8.9±1.3) was significantly lower than that of the nontransfusion group (n=21, 10.4±1.2). The most commonly transfused blood product intraoperatively was leuko-reduced filtered red blood cells, followed by fresh frozen plasma. When the study period was divided into two halves based on the time of operation, the first half showed a higher number of significant transfusions.
Conclusions
In most elective kidney transplant cases, surgery was conducted without the need for blood transfusion. The timing of transfusion, when necessary, shifted from during the operation to after the operation. The implementation of patient blood management, coupled with advancements in surgical techniques, appears to have impacted the pattern of perioperative transfusion.
Inventory management is necessary for blood banks to ensure an adequate supply of blood products, ensuring they meet patient needs promptly. This process involves monitoring the stock levels of various blood types and components, as well as keeping track of the shelf life of these blood products. Moreover, predicting the demand for transfusions is vital for effective and efficient blood product inventory management. For example, blood banks must maintain ample stocks of blood products due to the potential risk of emergency situations, such as massive bleeding during trauma or transplant surgery [1,2].
Kidney transplantation is a commonly performed operation with a high success rate. The outcomes for patients undergoing kidney transplantation have been significantly enhanced due to advancements in surgical techniques, immunosuppressants, and postoperative care. The current survival rate for transplant patients exceeds 90% after 1 year and 80% after 5 years [3]. As per the Organ Procurement and Transplantation Network statistics in the United States, the number of kidney transplants has seen a steady rise, from 17,728 cases in 2010 to 26,308 cases in 2022 [4]. Similarly, the Statistical Yearbook of the National Organ Blood Management Service reports an increase in the number of cases in Korea, from 491 to 677, during the same period [5].
Kidney transplant patients may require blood transfusions to compensate for blood lost during the procedure [6]. The volume of blood loss can vary, often significantly, particularly if the patient has concurrent medical conditions that hinder blood clotting. Patients receiving a transplant from a deceased donor may be at an increased risk of bleeding due to the organ preservation process [7,8]. Recently, however, the necessity for postoperative blood transfusions in kidney transplant patients has declined, thanks to advancements in surgical techniques and blood preservation methods, such as the use of cell savers. Despite this, the current global blood shortage [9-11] underscores the importance of having blood products readily available if needed. Given that kidney transplantation is a major surgery, the risk of substantial bleeding is always present. Our aim was to determine the frequency of blood transfusions in kidney transplant surgeries due to a lack of blood products, and to analyze the transfusion patterns in these patients. Additionally, we sought to investigate whether there has been a shift in these transfusion patterns over time.
We conducted this study in compliance with the principles of the Declaration of Helsinki. The study’s protocol was reviewed and approved by the Institutional Review Board of Hallym University Sacred Heart Hospital (IRB No. 2023-01-021-002). Informed consent was waived due to the retrospective nature of this study.
We conducted a retrospective descriptive study using 10 years’ worth of data. The data were sourced from a blood bank that supports an 830-bed tertiary care academic hospital in Anyang, Korea. Our aim was to analyze perioperative transfusion patterns in patients undergoing elective kidney transplantation. To this end, we gathered data from all complement-dependent cytotoxicity-crossmatching (CDC-XM) assays conducted between 2013 and 2022, a span of 10 years. We reviewed transfusion records, including the number of blood products used and any adverse reactions to transfusion. To compare the transfusion and nontransfusion groups, we collected preoperative and postoperative laboratory findings. These included measurements for hemoglobin concentration, platelet count, international normalized ratio (INR), and activated partial thromboplastin time (aPTT).
Statistical analyses were conducted using SPSS ver. 25 (IBM Corp.). The chi-square test was utilized to compare categorical variables. For continuous variables, we employed the Student t-test after verifying normality with the Shapiro-Wilk test. We used the Levene test to evaluate the equality of variance. A P-value of less than 0.05 was considered statistically significant.
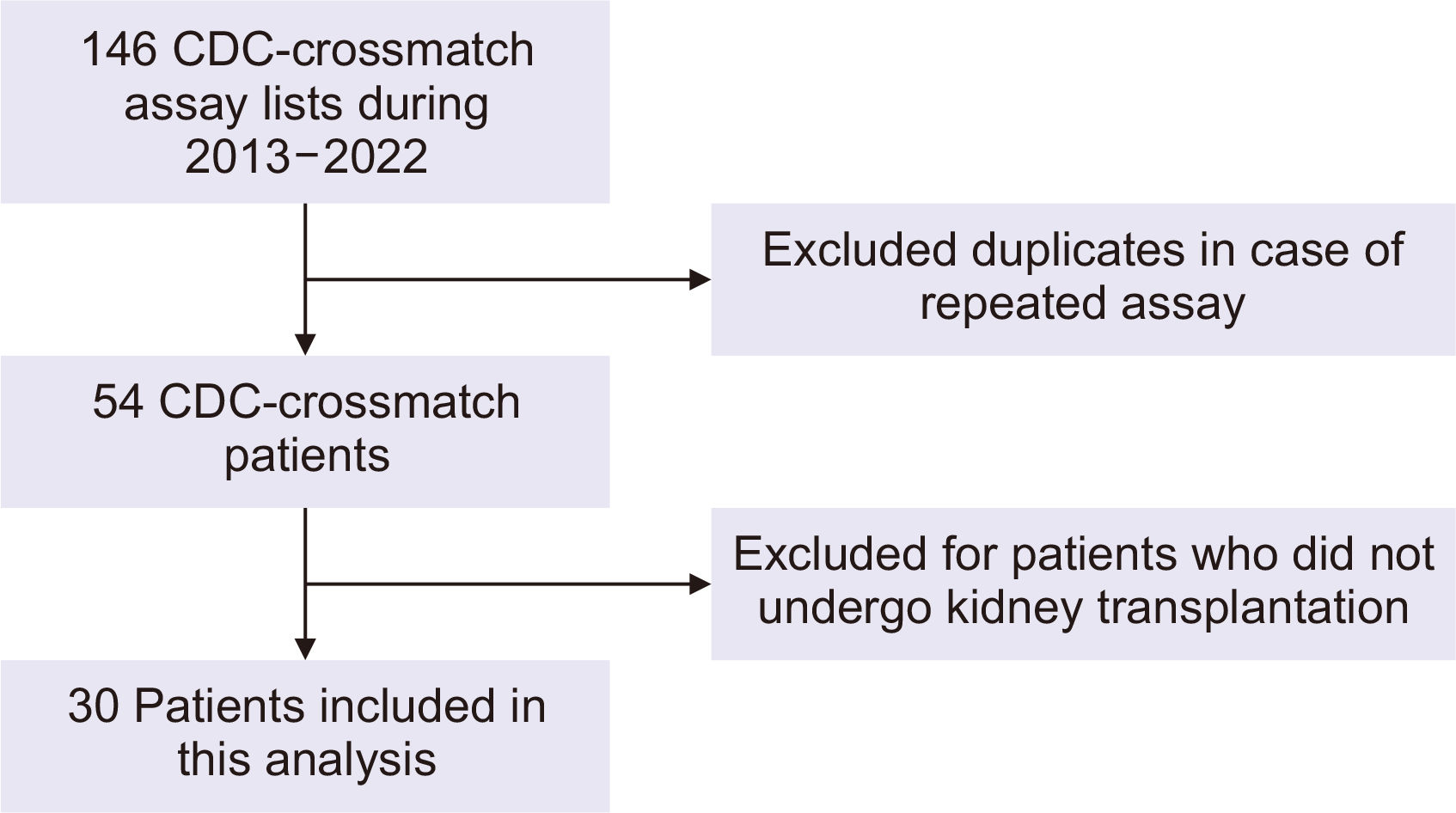
Between 2013 and 2022, the CDC-XM test was administered to 146 patients. The CDC-XM is a test that determines a patient's donor-specific antibodies by reacting the donor's lymphocytes with the patient's serum prior to kidney transplantation [12]. After excluding duplicate results from repeated assays, 54 patients remained. We excluded patients who did not undergo kidney transplantation surgery at our hospital. Ultimately, a total of 30 patients were included in this study (Fig. 1).
The mean age of the patients was 48.07 years, with a standard deviation of 9.7 (Table 1). The ratio of males to females was 1.5:1. Upon analyzing the CDC-XM results, it was found that T cell CDC-XM results were all negative, while B cell CDC-XM positive results were observed in eight patients. Four patients received blood transfusions during surgery, eight patients received blood transfusions after surgery, and three patients received blood transfusions during surgery and after surgery. Therefore, a total of nine patients received transfusion perioperatively. The average number of transfusion units (considering only red blood cells) per patient transfused during surgery was 2.5.
We divided the 30 patients into two groups: the transfusion group (n=9) and the nontransfusion group (n=21) (Table 2). There were no instances of graft loss in either the transfusion or nontransfusion groups. One patient in the nontransfusion group passed away, with the cause of death being gastrointestinal bleeding. The preoperative levels of hemoglobin, platelet count, INR, and aPTT did not significantly differ between the two groups. The postoperative hemoglobin level in the transfusion group (8.9±1.3) was significantly lower than that in the nontransfusion group (10.4±1.2, P=0.005). The postoperative platelet count, INR, and aPTT levels did not show significant differences between the two groups.
From the transfusion group, leuko-reduced filtered red blood cells were the most frequently transfused intraoperatively, with six units, followed by fresh frozen plasma with three units. Postoperatively, eight units of leuko-reduced filtered red blood cells were transfused, along with three units of fresh frozen plasma. The study period was divided into two halves according to the time of operation: the first half (n=15) and the second half (n=15). In the first half, seven out of 15 patients received a blood transfusion, while in the second half, only two out of 15 patients received a transfusion. There were significantly more transfusions in the first half of the period (P=0.046). Upon analyzing the transfusion records for both intraoperative and postoperative transfusions throughout the entire study period, no reports of adverse transfusion reactions were found.
Previous studies on kidney transplantation and transfusion have primarily concentrated on immune responses. Prior to the advent of immunosuppressants, blood transfusion was thought to have suppressive effects on various aspects of the immune system [13]. As such, blood products were transfused to patients in the hope that they would act as immunomodulators during transplantation surgeries. However, later studies revealed that transfusion actually increases the likelihood of sensitization to human leukocyte antigens, which can complicate transplantation [14]. To mitigate the risk of alloimmunization, it is now advised that blood transfusion be judiciously considered in the preoperative management of patients awaiting kidney transplantation [15]. Furthermore, the demand for blood transfusion following kidney transplantation has decreased due to advancements in surgical techniques and the implementation of blood conservation methods such as a cell-saver.
Despite concerted efforts to minimize blood transfusion during transplant surgeries, it remains crucial to have blood components readily available should they be required. Blood banks grapple with daily challenges, not only due to the constant need to prepare for emergency situations like unexpected massive bleeding during surgery, but also because chronic blood shortages are becoming increasingly common. This shortage of blood is a global issue, and it is a critical concern that needs to be addressed in Korea. As of September 2022, 17.8% of the Korean population is over 65 years old. The significant decrease in the blood donor population, largely due to demographic changes, poses a major problem for Korean society [16]. In such a scenario of blood shortage, it becomes necessary to accurately forecast the transfusion demand for major surgical procedures, such as kidney transplantation.
The maximum surgical blood order schedule (MSBOS) is a forecasting strategy designed to reduce unnecessary presurgical blood orders and associated costs in blood banks [17]. The MSBOS is calculated based on intraoperative blood usage over a previous period within each institution. In our study, a total of 10 units of red blood cell products were transfused intraoperatively into four patients, with the mean transfusion units per patient being 2.5. According to a previous study by Park et al. [18] in Korea, the number of patients receiving transfusions during kidney transplantation was 0.8%, with the mean units per transfused patient being 2.0. Consequently, the MSBOS for kidney transplantation was set at 2.0 [18]. Another study on MSBOS setting in the United States recommended that the number of crossmatching units prepared before kidney transplantation surgery should be set at 2.0 red blood cell units. The amount of blood products that can be transfused is expected to vary depending on the size of the institution, its infrastructure, the severity of the patients' conditions, and other factors. However, blood usage during kidney transplant surgery has shown a similar pattern.
The analysis of transfusion times revealed a shift from intraoperative to postoperative. It appears that the advent of patient blood management, coupled with advancements in surgical techniques, has influenced the pattern of blood transfusions before and after surgery. Depending on the timing, perioperative transfusions can be categorized as preoperative, intraoperative, or postoperative. The goal of perioperative transfusions is to maintain cardiac output volume support, enhance hemostasis, and boost oxygen-carrying capacity [19]. In the preoperative phase, laboratory tests were conducted to identify any underlying anemia in patients [20]. It is crucial to address the root cause of anemia before proceeding with surgery. During surgery, pharmacological treatments such as tranexamic acid and epsilon aminocaproic acid were administered to minimize perioperative blood loss. Other topical hemostatic agents, like fibrin sealant, were also utilized to aid in blood clotting [21]. In the postoperative phase, it is essential to continue efforts to prevent coagulopathy and reduce the risk of bleeding. To avoid iatrogenic anemia, blood samples should only be drawn when absolutely necessary. This study found that blood transfusions were significantly less frequent in the latter half of the analysis period compared to the first half. While this study does not analyze patient blood management, it seems to yield intriguing results.
This study has some limitations. The amount of transfusion required during surgery can vary depending on the severity of the patient's condition. However, this study only compared laboratory findings. A more robust analysis might be possible if factors such as estimated blood loss, surgery duration, prognosis, and patient severity are taken into account. Furthermore, this study's scope is limited due to the small patient sample from a single institution, which restricts the confirmation of statistical significance. The findings of this retrospective study may not be applicable to other hospitals with different blood bank policies and patient demographics, as it only investigated a single tertiary care center. Conducting retrospective multi-center research could potentially address these generalization concerns.
In our study, we found that most elective kidney transplantations were performed without the need for a blood transfusion. Furthermore, when transfusions were necessary, the timing typically shifted from the intraoperative to the postoperative phase. The incorporation of patient blood management principles and advancements in surgical techniques seem to have significantly influenced this transfusion trend during the perioperative period. Blood transfusions are now administered with greater care and consideration, contributing to improved patient recovery and exemplifying the increasing precision in medical practice.
Acknowledgments
Yun Joo Kang (Hallym Sacred Heart Hospital, Anyang, Korea) provided data collection.
ARTICLE INFORMATION
Author Contributions
Conceptualization: HJK. Data curation: SP. Formal analysis: JR. Funding acquisition: HJK. Investigation: SP. Methodology: HJK. Project administration: HJK. Visualization: JR. Writing–original draft: JR. Writing–review & editing: all authors. All authors read and approved the final manuscript.
REFERENCES
1. Guo C, Gong M, Ji L, Pan F, Han H, Li C, et al. 2022; A prediction model for massive hemorrhage in trauma: a retrospective observational study. BMC Emerg Med. 22:180. DOI: 10.1186/s12873-022-00737-y. PMID: 36376795. PMCID: PMC9661746.
2. Guduri PR, Shastry S, Raturi M, Shenoy A. 2022; Surgical blood ordering schedule for better inventory management: an experience from a tertiary care transfusion center. Med J Armed Forces India. 78:283–90. DOI: 10.1016/j.mjafi.2020.07.004. PMID: 35855717. PMCID: PMC9287726.
3. Hariharan S, Israni AK, Danovitch G. 2021; Long-term survival after kidney transplantation. N Engl J Med. 385:729–43. DOI: 10.1056/NEJMra2014530. PMID: 34407344.
4. Organ Procurement and Transplantation Network (OPTN). 2023. National OPTN data [Internet]. OPTN;Available from: https://insights.unos.org/OPTN-metrics/. cited 2023 Jan 19.
5. Korean Network for Organ Sharing (KONOS). 2020. 2020 Annual report of organ and tissue donation and transplantation [Internet]. KONOS;Available from: https://www.konos.go.kr/board/boardListPage.do?page=sub4_2_2&boardId=22. cited 2023 Jan 19.
6. Bynum JP, Zachary A, Ness PM, Luo X, Bagnasco S, King KE, et al. 2018; Transfusion of leukoreduced blood products and risk of antibody-mediated rejection of renal allografts. Transfusion. 58:1951–7. DOI: 10.1111/trf.14800. PMID: 30171817. PMCID: PMC6131050.
7. Amara D, Parekh J, Sudan D, Elias N, Foley DP, Conzen K, et al. 2022; Surgical complications after living and deceased donor liver transplant: the NSQIP transplant experience. Clin Transplant. 36:e14610. DOI: 10.1111/ctr.14610. PMID: 35143698.
8. Scornik JC, Schold JD, Bucci M, Meier-Kriesche HU. 2009; Effects of blood transfusions given after renal transplantation. Transplantation. 87:1381–6. DOI: 10.1097/TP.0b013e3181a24b96. PMID: 19424040.
9. Jacobs JW, Booth GS. 2022; Blood shortages and changes to massive transfusion protocols: survey of hospital practices during the COVID-19 pandemic. Transfus Apher Sci. 61:103297. DOI: 10.1016/j.transci.2021.103297. PMID: 34690073. PMCID: PMC8530788.
10. Kim KW, Kim OS. 2020; Super aging in South Korea unstoppable but mitigatable: a sub-national scale population projection for best policy planning. Spat Demogr. 8:155–73. DOI: 10.1007/s40980-020-00061-8. PMID: 34222615. PMCID: PMC8248505.
11. Loudon AM, Rushing AP, Hue JJ, Ziemak A, Sarode AL, Moorman ML. 2023; When is enough enough? Odds of survival by unit transfused. J Trauma Acute Care Surg. 94:205–11. DOI: 10.1097/TA.0000000000003835. PMID: 36694331.
12. Mahowald GK. 2021; The CDC crossmatch in the era of flow cytometric cross-match and single antigen beads. J Bras Nefrol. 43:299–300. DOI: 10.1590/2175-8239-jbn-2021-0110. PMID: 34237125. PMCID: PMC8428642.
13. George CD, Morello PJ. 1986; Immunologic effects of blood transfusion upon renal transplantation, tumor operations, and bacterial infections. Am J Surg. 152:329–37. DOI: 10.1016/0002-9610(86)90269-2. PMID: 3530001.
14. Obrador GT, Macdougall IC. 2013; Effect of red cell transfusions on future kidney transplantation. Clin J Am Soc Nephrol. 8:852–60. DOI: 10.2215/CJN.00020112. PMID: 23085723.
15. da Silva SF, Ferreira GM, da Silva SL, Alves TM, Ribeiro IF, Ribeiro TR, et al. 2013; Red blood cell and leukocyte alloimmunization in patients awaiting kidney transplantation. Rev Bras Hematol Hemoter. 35:185–8. DOI: 10.5581/1516-8484.20130043. PMID: 23904808. PMCID: PMC3728131.
16. Kim HO. 2022; Current state of blood management services in Korea. Ann Lab Med. 42:306–13. DOI: 10.3343/alm.2022.42.3.306. PMID: 34907100. PMCID: PMC8677471.
17. Guzman JP, Resurreccion LL, Gepte MB. 2019; Use of Maximum Surgical Order Schedule (MSBOS) among pediatric patients to optimize blood utilization. Ann Pediatr Surg. 15:4. DOI: 10.1186/s43159-019-0005-9.
18. Park JR, Alghamdi E, Kim S, Kim HO. 2015; An update of maximum surgical blood order schedule in elective surgery. Korean J Blood Transfus. 26:38–46. DOI: 10.17945/kjbt.2015.26.1.38.
19. Ferraris VA, Ferraris SP, Saha SP, Hessel EA 2nd, Haan CK, et al. Society of Thoracic Surgeons Blood Conservation Guideline Task Force. 2007; Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 83(5 Suppl):S27–86. DOI: 10.1016/j.athoracsur.2007.02.099. PMID: 17462454.
20. Desai N, Schofield N, Richards T. 2018; Perioperative patient blood management to improve outcomes. Anesth Analg. 127:1211–20. DOI: 10.1213/ANE.0000000000002549. PMID: 29064875.
21. Carless PA, Henry DA, Anthony DM. 2003; Fibrin sealant use for minimising peri-operative allogeneic blood transfusion. Cochrane Database Syst Rev. 2003:CD004171. DOI: 10.1002/14651858.CD004171. PMCID: PMC4171968.
Table 1
Clinical characteristics of the patients analyzed in this study
Table 2
Laboratory findings of the kidney transplantation patients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download