INTRODUCTION
From January 2011 to December 2017, 83 patients with end-stage liver disease caused by biliary atresia were referred to Dr. Cipto Mangunkusumo Hospital (CMH) [
1], and 282 patients with hepatocellular carcinoma (HCC) were referred to CMH or Dharmais National Cancer Hospital between January 2015 and November 2017. The mortality rate for HCC reached 48.2% [
2]. The most common indication for liver transplantation (LT) in Indonesia was biliary atresia (in children) and HCC due to hepatitis B or C infection (in adults) [
3].
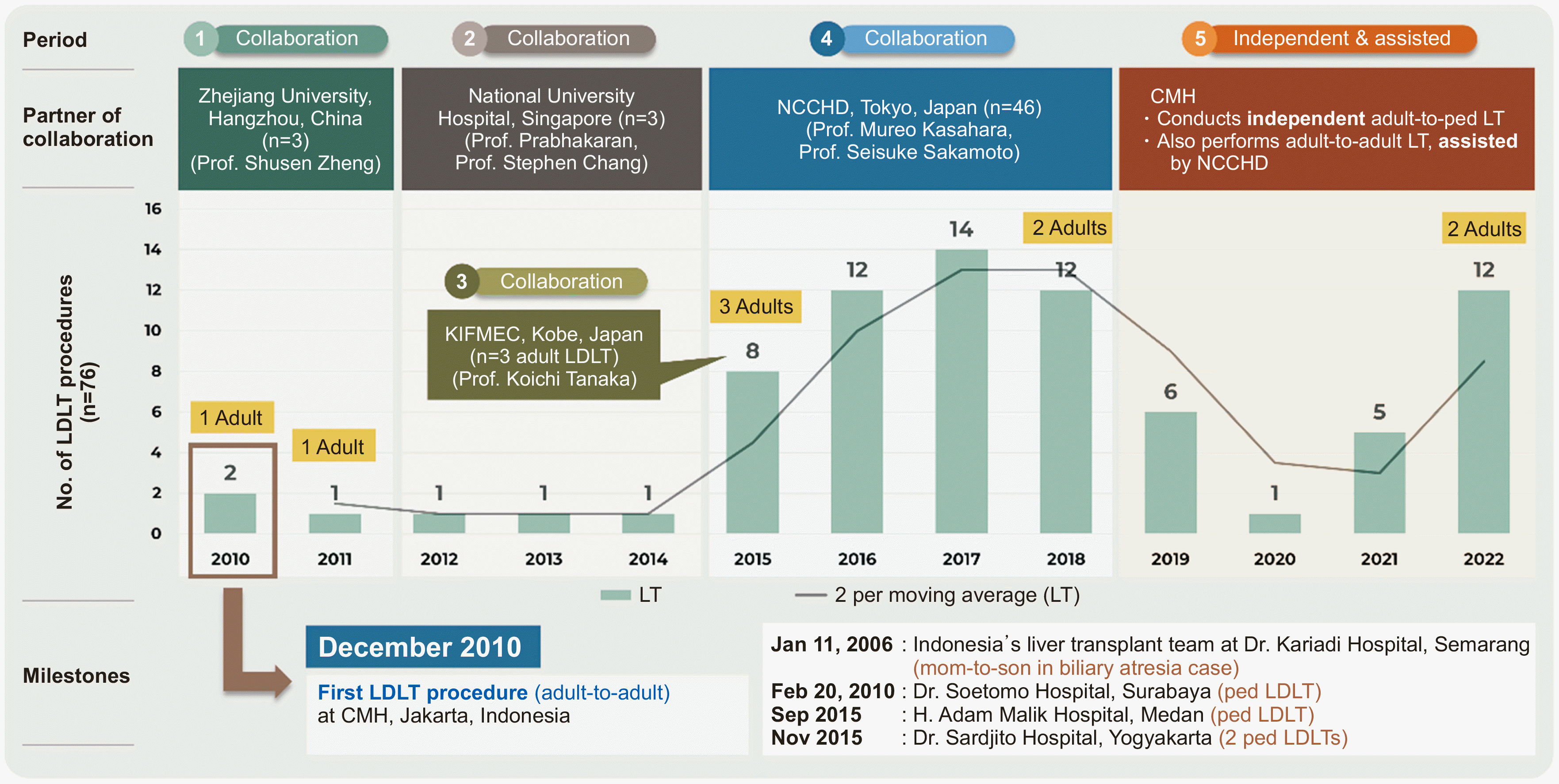
The living donor LT (LDLT) initiative in Indonesia began in 2006. As the Indonesian national referral center and a tertiary-level hospital, CMH realized this initiative in December 2010 by performing the first adult-to-adult LDLT in collaboration with Zhejiang University, Hangzhou, China. Today, CMH is the only center that routinely performs LT in Indonesia [
3]. In 2012–2014, CMH continued its partnership journey with National University Hospital, Singapore. Then, in 2015 the institution successfully conducted three adult-to-adult LDLTs while receiving assistance from the Kobe International Frontier Medical Center in Japan. From 2015 to 2018, the Japanese National Center for Child Health and Development (NCCHD) took over this collaboration agreement. Currently, CMH independently conducts adult-to-pediatric LT and performs assisted adult-to-adult LT [
3]. A timeline of the history of LDLT programs at CMH is presented in
Fig. 1.
Fig. 1
Timeline for providing liver transplantation services in Indonesia by Dr. Cipto Mangunkusumo Hospital (CMH)’s liver transplantation team. NCCHD, National Center for Child Health and Development; ped, pediatric; LT, liver transplantation; LDLT, living donor LT; KIFMEC, Kobe International Frontier Medical Center.

All LTs in Indonesia have been conducted using living liver donors (LLDs). Despite approval by religious and governmental authorities, deceased donor LT (DDLT) has not become a preferred source of donor organs [
2,
3]. This may be associated with the lack of a donor registration system (like the existing Eye Bank in Indonesia) and with the low public awareness of the LT program. Therefore, the only effective procedure conducted in Indonesia has been LDLT in terms of transplant team skills, available facilities, and material support.
The characteristics of LLDs in Indonesia have not been documented. Knowledge of donors is important in developing an LDLT program, assisting the government in policy-making, helping the national transplantation committee create national guidelines for donor selection criteria, and supporting other centers that wish to build their own transplantation service. This study presents current updates on the clinical characteristics, graft types, and intraoperative and postoperative characteristics of LDLT after 13 years of experience at our institution.
METHODS
Ethical approval was issued by the Institutional Review Board (IRB) of the Health Research Ethics Committee of the Faculty of Medicine Universitas Indonesia and CMH (IRB protocol No. 22-04-0479). Informed consent was not required because all data were collected from the LT registry. The study conformed to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (
Supplementary Table 1) [
4].
This was a retrospective descriptive study. All data were collected from the CMH LT registry, as the only active LT center in Indonesia, from January 1, 2010 to December 31, 2022.
Subjects and Donor Selection
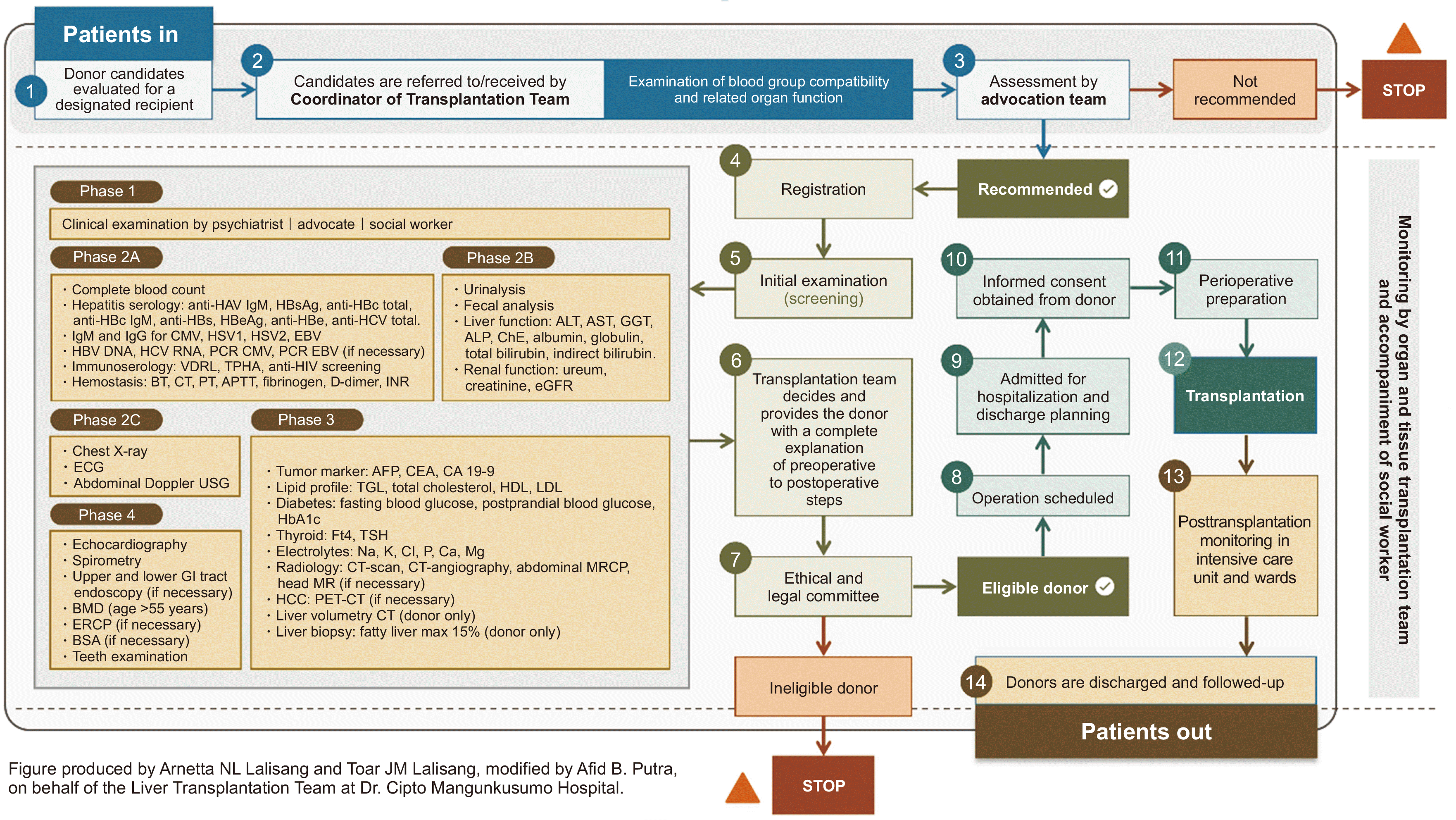
We included all LLDs who participated in the LDLT program at CMH. All LLD subjects were included without exclusions, which could introduce biases. For example, most procedures were adult-to-pediatric, which affected the type of liver graft chosen. A total of 76 LLDs received postoperative care at CMH. The living donor screening and selection began 30 days before a procedure. The pathway of the 14-step process is shown in
Fig. 2. This pathway was designed to evaluate the donor’s risk to ensure their safety and well-being. More than one donor could undergo this assessment for each recipient. The screening form, including all required phases, is presented in
Supplementary Material 1.
Fig. 2
Donor selection pathway and examinations that must be completed before performing a transplantation procedure at Dr. Cipto Mangunkusumo Hospital. HAV, hepatitis A virus; IgM, immunoglobulin M; HBs, hepatitis B surface; Ag, antigen; HBc, hepatitis B core; HBe, hepatitis B envelope; HCV, hepatitis C virus; IgG, immunoglobulin G; CMV, cytomegalovirus; HSV, herpes simplex virus; EBV, Epstein-Barr virus; HBV, hepatitis B virus; HCV, hepatitis C virus; RNA, ribonucleic acid; PCR, polymerase chain reaction; VDRL, venereal disease research laboratory; TPHA, treponema pallidum hemagglutination; HIV, human immunodeficiency virus; BT, bleeding time; CT, clotting time (Phase 2A); PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; ChE, serum cholinesterase; eGFR, estimated glomerular filtration rate; ECG, electrocardiogram; USG, ultrasonography; AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9; TGL, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, hemoglobin A1C; FT4, free thyroxine; TSH, thyroid stimulating hormone; CT, computed tomography; MRCP, magnetic resonance cholangiopancreatography; MR, magnetic resonance; HCC, hepatocellular carcinoma; PET, positron emission tomography; GI, gastrointestinal; BMD, bone mineral densitometry; ERCP, endoscopic retrograde cholangiopancreatography; BSA, body surface area.

Variables
This study focused exclusively on data regarding the clinical, graft, and intraoperative and postoperative characteristics of the LLDs. Clinical characteristics included the liver recipient’s age and the donor’s age, body mass index (BMI), relationship to the recipient, sex, comorbidities, blood type, and ABO compatibility. Graft characteristics included remnant liver volume (RLV), total liver volume (TLV), RLV:TLV ratio, estimated graft weight (EGW) according to computed tomography (CT), actual graft weight (AGW), actual:EGW ratio, graft-to-recipient weight ratio (GRWR), graft types, fatty liver status, and variations of the vascular-biliary structure (biliary duct based on the Huang classification, the hepatic artery based on the Michels classification, hepatic portal vein based on the Nakamura classification, and hepatic vein variations). The intraoperative and postoperative characteristics included the operation time, parenchymal transection time, blood loss volume, intensive care unit (ICU) length of stay (LOS), total LOS, and complications after surgery based on the Clavien-Dindo classification.
Data Analysis
Continuous variables were presented as mean±standard deviation or median (range), while categorical variables were presented as frequencies and percentages. Data normality was calculated using the Kolmogorov-Smirnov test, with P>0.05 indicating normally distributed data. All analyses were performed using SPSS ver. 20 (IBM Corp.).
RESULTS
The yearly number of LT procedures fluctuated, with the highest numbers occurring between 2015 and 2018. The overall mean was 5.8 procedures per year; however, this number has increased to 8.8 procedures per year over the last 8 years. The number of LDLT procedures increased in line with our intensive collaboration with NCCHD in Japan. Two professors came to Indonesia and physically assisted in our operations. After routinely performing two to three procedures per month, we now independently perform adult-to-pediatric LDLTs. However, they are still assisting us in adult-to-adult LDLT.
Adult-to-pediatric LDLT has been conducted more frequently than adult-to-adult LDLT (88% vs. 12%, respectively). Of the 76 LLDs, the average age was 31.8±6.1 years. Most LLDs were aged 30 to 39 years (52%), were parents of the recipients (mothers, 49%; fathers, 38%), and were female 59%. In total, 27 LLDs (36%) had comorbidities, including diabetes mellitus (7%), hypertension (4%), smoking (15%), and a history of cesarean section (14%). Other comorbidities included alcohol consumption, uterine myoma, mammary fibroadenoma, and a history of appendectomy. Other clinical characteristics of the LLDs are presented in
Table 1.
Table 1
Clinical characteristics of living liver donors at Dr. Cipto Mangunkusumo Hospital, Indonesia from January 1, 2010 to December 31, 2022
|
Donor characteristics |
Total subjects (n=76) |
Adult recipients (n=9) |
Pediatric recipients (n=67) |
|
LDLT type |
|
|
|
|
Adult-to-pediatric |
67 (88.2) |
|
|
|
Adult-to-adult |
9 (11.8) |
|
|
|
Age (yr) |
31.8±6.1 |
31.1±9.8 |
31.9±5.6 |
|
Donor age group (yr) |
|
|
|
|
18–29 |
29 (38.2) |
4 (44.4) |
25 (37.3) |
|
30–39 |
39 (51.3) |
3 (33.3) |
36 (53.7) |
|
40–50 |
8 (10.5) |
2 (22.3) |
6 (9.0) |
|
Relationship to recipient |
|
|
|
|
First-degree relative (parents) |
68 (89.6) |
5 (55.6) |
63 (94.0) |
|
Other blood relative |
|
|
|
|
Uncle/aunt |
4 (5.2) |
- |
4 (6.0) |
|
Nephew |
1 (1.3) |
1 (11.1) |
- |
|
Husband/wife |
2 (2.6) |
2 (22.2) |
- |
|
Other |
1 (1.3) |
1 (11.1) |
- |
|
Sex |
|
|
|
|
Female |
45 (59.2) |
6 (66.7) |
39 (58.2) |
|
Male |
31 (40.8) |
3 (33.3) |
28 (41.8) |
|
BMI Asia-Pacific (kg/m2) |
22.5±2.7 |
|
|
|
BMI Asia-Pacific group (kg/m2) |
|
|
|
|
Underweight (<18.5) |
8 (10.5) |
- |
8 (11.9) |
|
Normal (18.5–22.9) |
34 (44.7) |
4 (44.4) |
30 (44.8) |
|
Overweight (23–24.9) |
19 (25.0) |
3 (33.3) |
16 (23.9) |
|
Obese (≥25.0) |
15 (19.8) |
2 (22.2) |
13 (19.4) |
|
Donor comorbidity |
|
|
|
|
No |
49 (64.5) |
6 (66.7) |
43 (64.2) |
|
Yes |
27 (35.5) |
3 (33.3) |
24 (35.8) |
|
Donor’s blood type |
|
|
|
|
A+ |
13 (17.1) |
2 (22.2) |
12 (17.9) |
|
B+ |
26 (34.2) |
5 (55.6) |
21 (31.3) |
|
AB+ |
3 (3.9) |
- |
3 (4.5) |
|
O+ |
34 (44.7) |
2 (22.2) |
32 (47.8) |
|
ABO compatibility |
|
|
|
|
Incompatible |
4 (5.3) |
1 (11.1) |
3 (4.5) |
|
Compatible |
72 (94.7) |
8 (88.9) |
64 (95.5) |

The graft types are shown in
Table 2. Most grafts were from left lateral sectionectomy (86%); without fatty liver (90%); classified as biliary duct Huang A1 (n=30, 60.0%), hepatic artery Michels I (n=52, 74.2%), and portal vein Nakamura A (n=67, 88.2%); and used hepatic veins with a common left and middle trunk (n=46, 60.5%). Other variations of the hepatic vein are depicted in
Fig. 3.
Fig. 3
List of other hepatic vein variations other than the ones previously appeared in
Fig. 2. LHV, left hepatic vein; MHV, middle hepatic vein; RHV, right hepatic vein; IVC, inferior vena cava.
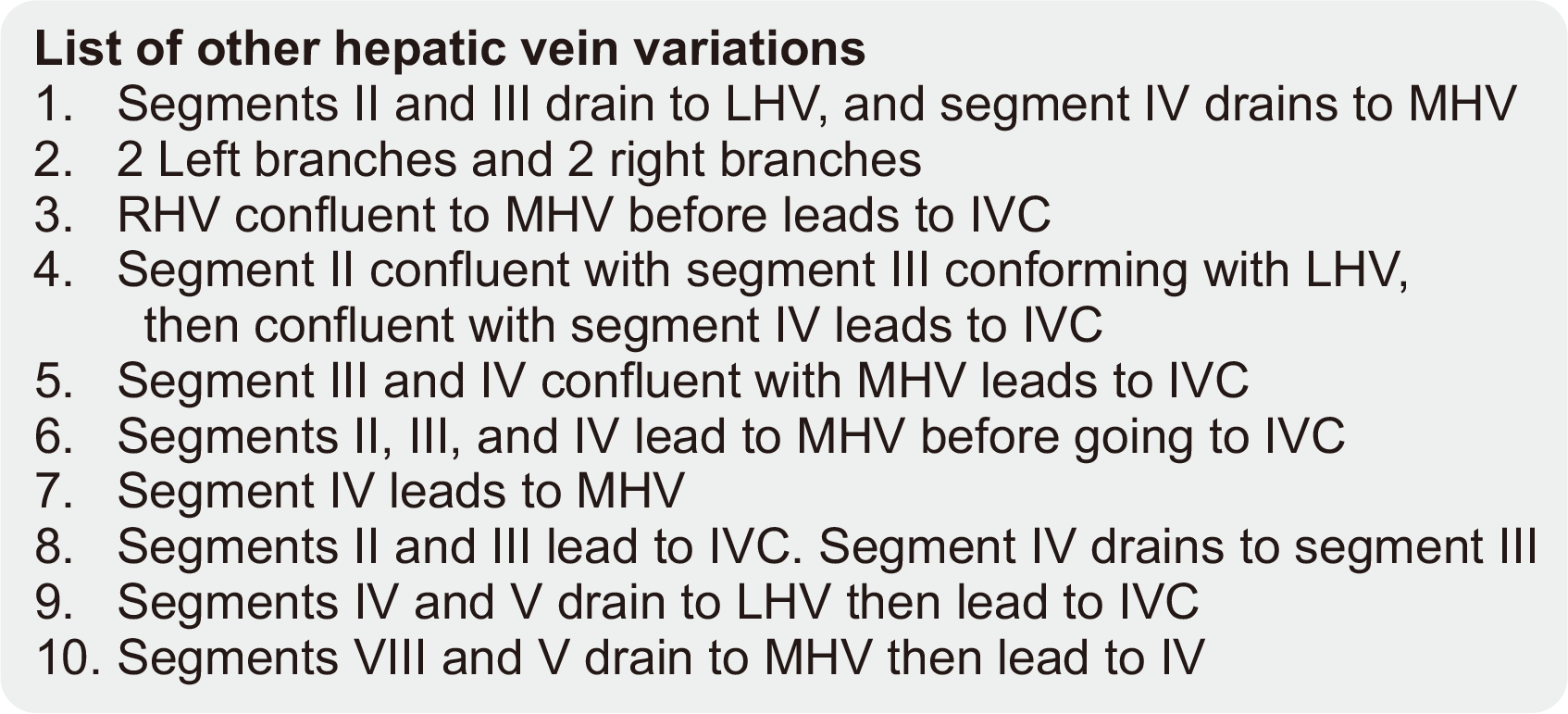

Table 2
Graft characteristics in living donor liver transplantation at Dr. Cipto Mangunkusumo Hospital in Indonesia from January 1, 2010 to December 31, 2022
|
Characteristics of donor graft |
Total subjects (n=76) |
Adult recipients (n=9) |
Pediatric recipients (n=67) |
|
Graft types |
|
|
|
|
Left lateral (II, III) |
65 (85.5) |
1 (11.1) |
64 (95.5) |
|
Left lobe (II, III, IV) |
4 (5.3) |
1 (11.1) |
3 (4.5) |
|
Left lobe+segment I |
3 (3.9) |
3 (33.3) |
- |
|
Right lobe |
4 (5.3) |
4 (44.5) |
- |
|
Remnant liver volume (mL/m2) |
976.5 (564.6–1,957) |
893.4 (564.6–980.5) |
997.6 (750.9–1,957) |
|
Total liver volume (mL/m2) |
1,251 (976.8–2,317) |
1,285 (1,184–1,680) |
1,249 (976.8–2,317) |
|
RLV:TLV ratio (%) |
79.5 (47.7–85.8) |
69.5 (47.7–71.2) |
80.3 (66.3–85.8) |
|
CT-estimated GW (g) |
248.4 (122.8–787.5) |
484.6 (297.9–787.5) |
243.9 (122.8–500.4) |
|
Actual GW (g) |
255 (149–700) |
510 (360–700) |
240 (149–430) |
|
Actual:estimated GW ratio (%) |
104.8±21.2 |
106.5±25.7 |
104.7±20.9 |
|
CT graft-to-recipient weight ratio (%) |
2.69 (0.46–7.91) |
0.83 (0.46–1.43) |
2.80 (1.02–7.91) |
|
Actual graft-to-recipient weight ratio (%) |
2.65±1.21 |
0.84±0.19 |
2.89±1.07 |
|
Fatty liver status |
|
|
|
|
No |
68 (89.5) |
7 (77.8) |
61 (91.0) |
|
Mild |
6 (7.9) |
2 (22.2) |
4 (6.0) |
|
Moderate |
2 (2.6) |
- |
2 (3.0) |
|
Biliary duct variations |
|
|
|
|
Huang A1 |
30 (60.0) |
NA |
NA |
|
Huang A2 |
8 (16.0) |
|
|
|
Huang A3 |
6 (12.0) |
|
|
|
Huang A4 |
5 (10.0) |
|
|
|
Huang A5 |
1 (2.0) |
|
|
|
Hepatic artery variations (Michels) |
|
|
|
|
I |
52 (74.2) |
7 (77.8) |
45 (73.8) |
|
II |
4 (5.7) |
- |
4 (6.6) |
|
III |
5 (7.1) |
1 (11.1) |
4 (6.6) |
|
IV |
2 (2.9) |
- |
2 (3.3) |
|
V |
2 (2.9) |
- |
2 (3.3) |
|
VI |
3 (4.3) |
- |
3 (4.8) |
|
IX |
2 (2.9) |
1 (11.1) |
1 (1.6) |
|
Portal vein variations (Nakamura) |
|
|
|
|
A |
67 (88.2) |
8 (88.9) |
59 (88.0) |
|
B |
5 (6.6) |
- |
5 (7.5) |
|
C |
4 (5.7) |
1 (11.1) |
3 (4.5) |
|
Hepatic vein variations |
|
|
|
|
RHV, MHV, and LHV leading to IVC |
13 (17.1) |
3 (33.3) |
10 (14.9) |
|
Common trunk (MHV confluent to LHV) |
46 (60.5) |
4 (44.5) |
42 (62.7) |
|
Segment IV draining to IVC |
3 (3.9) |
- |
3 (4.5) |
|
Segment IV draining to LHV |
2 (2.6) |
- |
2 (3.0) |
|
Segment IV draining to MHV |
1 (1.3) |
1 (11.1) |
- |
|
Othera)
|
11 (14.6) |
1 (11.1) |
10 (14.9) |

We analyzed differences between the CT-EGW and the AGW. The median EGW and AGW in the pediatric recipient group were 243.9 g (range, 122.8–500.4 g) and 240.0 g (range, 149.0–430.0 g), respectively. The median weight difference was 11.7 g (range, −180.4 to 128.3 g) and the percentage error was −4.9% (range, −41.7% to 56.4%). Meanwhile, the median EGW and AGW in the adult recipient group was 484.6 g (range, 297.9–787.5 g) and 510.0 g (range, 360.0–700.0 g), respectively, with a median weight difference of 38.1 g (range, −217.5 to 116.8 g) and a percentage error of −7.6% (range, −27.1% to 38.2%).
We also calculated the estimated GRWR (EGRWR) and actual GRWR (AGRWR). The median EGRWR and AGRWR for the adult-to-pediatric group were 2.80% (range, 1.02%–7.91%) and 2.86% (range, 0.84%–6.19%), respectively. Meanwhile, the median EGRWR and AGRWR for the adult-to-adult group were 0.83% (range, 0.46%–1.43%) and 0.74% (range, 0.63%–1.09%), respectively.
The intraoperative and postoperative characteristics are presented in
Table 3. The median ICU LOS and total LOS were 2 days (range, 1–5 days) and 7 days (range, 4–28 days), respectively, and the overall complication rate was 23% (n=18). The common complications were fever (7/76) and gastrointestinal immobility (6/76). Other reported complications included bile leakage (n=2), bile duct stenosis (n=1), pulmonary embolism (n=1), pleural effusion (n=2), hospital-acquired pneumonia (n=2), surgical site infection (n=3), transfusion allergic reaction (n=1), and postoperative anxiety (n=4). The postoperative anxieties were related to postsurgical wound incisions, regret after donating a liver, and concerns about the recovery of liver function. All complications were successfully treated (excluding postoperative anxiety) and no donor mortality was reported. All donors returned home fully recovered based on their clinical status. However, records for the improvement of their anxiety were not available in our registry.
Table 3
Intraoperative and postoperative characteristics in living donor liver transplantation at Dr. Cipto Mangunkusumo Hospital in Indonesia from January 1, 2010 to December 31, 2022
|
Characteristics |
Total operations (n=76) |
Right lobe graft (n=4) |
Left lateral graft (n=65) |
Left lobe graft (n=7) |
|
Operation time (min) |
410 (200–756) |
509 (417–600) |
399.5 (200–756) |
500 (359–704) |
|
Parenchymal transection time (min) |
147 (64–324) |
132 (111–160) |
143 (6–324) |
182.0 (93–281) |
|
Blood loss volume (mL) |
300 (70–1,500) |
475 (350–600) |
300 (70–1,300) |
400 (100–1,500) |
|
ICU LOS (day) |
2 (1–5) |
1.5 (1–2) |
2 (1–4) |
2 (1–5) |
|
Total LOS (day) |
7 (4–28) |
6.5 (6–7) |
7 (4–9) |
7 (4–28) |
|
Complicationsa)
|
|
|
|
|
|
Grade 1 |
50 (65.8) |
3 (75.0) |
42 (64.6) |
5 (71.4) |
|
Grade 2 |
25 (32.9) |
1 (25.0) |
22 (33.8) |
2 (28.6) |
|
Grade 3a |
- |
- |
- |
- |
|
Grade 3b |
1 (1.3) |
- |
1 (1.6) |
- |
|
Grade 4 |
- |
- |
- |
- |
|
Grade 5 |
- |
- |
- |
- |

DISCUSSION
This study showed that most grafts for LDLT in Indonesia came from young adults. This may be influenced by the average marriage age of 25 years in Indonesia, so that a parent’s age would be around 30 to 35 years when their child might need a transplanted liver. Donors aged 18–29 years had the best graft outcomes, likely due to their superior regenerative capacity, higher compliance, and lower vascular resistance, thus decreasing postoperative ascites rates [
5,
6].
Most subjects had an ideal BMI. Although several transplant centers reject donors with a BMI over 30 kg/m
2 [
7,
8], safe outcomes are still possible if the assessment shows minimal hepatic steatosis (<10%) [
9,
10]. An ideal BMI had no effect on the rate of fatty liver disease, but an obese BMI significantly increased the risk of fatty liver disease for each unit (kg/m
2) increase. Donors with higher BMIs had more adipose tissue and more fatty acids flowing to the liver [
11]. This implies that an overweight or obese status should not be an absolute contraindication to liver donation if the risk of steatosis is low.
The most common types of grafts in our study were left liver grafts (left lateral segment grafts [II, III] and left lobe grafts [II, III, IV]) due to the high number of pediatric liver transplant recipients in our institution. Even though their small graft volume limits their use to children and small adults, left liver grafts also pose a minimal risk to the donor [
12]. Only four subjects were right liver donors in this study. The left lobe liver volume may not be sufficient for the metabolic needs of an adult recipient. Therefore, a graft including the left lobe and the caudate lobe can be an alternative to increase its volume [
12].
Our investigation found no significant difference between EGWs and AGWs. Trends of underestimation or overestimation may be attributed to the type of software used to estimate liver volume [
13-
15]. The use of conventional CT versus semi-automatic three-dimensional CT volumetry might give different results [
16,
17]. However, differences in graft weight (GW) estimation of ±5% to ±20% were quite common [
13,
14,
16]. We recommend that each LT team learn the characteristics of their center’s diagnostic tools to be prepared for any discrepancies in the estimation of GW [
16].
We evaluated bile duct variations using preoperative magnetic resonance cholangiopancreatography (MRCP) and three instances of intraoperative cholangiography (IOC). The first IOC was performed before liver transection began, the second IOC was performed just before cutting the blood vessels and bile duct (after exposing all vasculature and bile ducts), and the last IOC was performed after parenchymal transection was finished. The steps were initiated by inflow occlusion, then followed by outflow occlusion. We used a Cavitron Ultrasonic Surgical Aspirator (CUSA) for liver parenchymal transection. CUSA is the most widely used device for parenchymal transection to avoid vascular injury during open surgery [
18].
One near-miss event, slippage of the vascular clamp, occurred in the left hepatic vein due to a clip that was too small to completely clamp the vessel. However, this situation was resolved quickly with minimal blood loss, and the liver was resected and the graft was taken out. We did not find any previously reported iatrogenic incidents. We did not perform postoperative magnetic resonance imaging (MRI) if the patient was clinically stable and was without postoperative jaundice or stricture. There was one reported complication of bile leakage during hepatectomy, but it was managed well. One case needed a relaparotomy, and four cases of surface leakage were managed conservatively by using adequate drainage until they spontaneously healed.
The duration of surgery in this study ranged from 10–13 hours, though it can be longer if significant blood loss (>1,000 mL) occurs. The duration and the amount of blood loss in our study were similar to a previous study [
19,
20]. We recommend the use of fibrin sealant and matrix coagulant sheets to help control bleeding during liver surgery [
21]. Transection coagulation techniques with bipolar forceps or soft coagulation using monopolar electrodes can also be used [
19].
The median LOS in this study was 7 days (range, 4–28 days), which is comparable to other studies where the LOS ranged from 6 to 38 days, with a mean of 22 days [
20]. The longest LOSs in our study occurred in the earliest period (2010–2016), but improved to only 6–7 days in the last 6 years after our collaboration with the Japanese institution. Regarding recipient outcomes, 22 of 67 pediatric recipients and six of nine adult recipients did not survive. During the collaboration (aided) period, 15 of 43 pediatric recipients and six of nine adult recipients died. However, this number has improved, and the outcomes are better today. During the subsequent independent period, only seven of 24 pediatric recipients have not survived. We are not yet performing adult-to-adult LDLT independently. Overall, the 1-year recipient survival rate was 84%, and the 5-year survival rate was 77%. The recipient mortality rate was 25%. The causes of recipient death were sepsis (3/4 cases), hemorrhagic shock (1/8 cases), and other (1/8 cases). The most reported complications were bile leakage, sepsis, bleeding, and acute cellular rejection.
CMH is classified as a low-volume center (<20 LTs/year) compared to other Asian countries such as India, Korea, Japan, China, Egypt, or Saudi Arabia, which perform hundreds of LDLT procedures each year [
22,
23]. The LT program in Indonesia developed later than those in other Southeast Asian countries, such as Malaysia, Thailand, and Singapore. The success of the LT program in Singapore is due to its lengthy history, dating back to 1990, which is 20 years earlier than in Indonesia [
24]. Thailand began its DDLT program in 1987 and its LDLT program in 2009 [
25]. Meanwhile, Malaysia started its LT program in 1995 [
26].
One factor that arises in low-volume LT centers is a shortage of LLDs, leading to long waiting lists for recipients [
23]. To overcome this problem, the use of DDLT is encouraged. While the reputation of Asia as a global leader in LDLT and a pioneer in developing LT techniques, DDLT is not as popular as LDLT for cultural, religious, socioeconomic, and policy reasons. However, Hibi et al. [
22] reported that DDLT is increasing in Korea and China, followed by Iran and India.
The number of LDLT procedures is increasing every year. CMH routinely schedules transplants approximately once per week. However, the number of adult-to-adult procedures is still far less than adult-to-pediatric procedures because the availability of donors for adult recipients is still low. At least five donor candidates are typically screened before one donor is selected that matches the recipient. In contrast, pediatric patients automatically have two donor candidates available, the recipient's father and mother, which usually suffices. In adult-to-pediatric LDLT, the parents are usually very open to donating their liver, whereas in adult-to-adult LDLT, most recipients refuse offers from their donor candidates.
The low number of potential liver donor candidates in Indonesia can be resolved with various approaches, including opening volunteer liver donor programs (such as the Indonesian Eye Bank system, or the United Network for Organ Sharing in the United States [US]), issuing policies for implementing LDLT services outside of CMH, creating national guidelines for implementing liver transplants with related criteria for the selection and allocation of donors (such as the Organ Procurement and Transplantation Network in the US), promoting the DDLT program, increasing awareness of the importance of liver donations, giving awards to donors or their heirs, and allocating health coverage to LLDs for the screening process and surgery [
27]. The Indonesian national health insurance only covers 269 million rupiah, out of a total cost of up to 600 million rupiah for LDLT [
28]. Costs for liver donors must be borne out-of-pocket by the recipient's family or crowdfunding (open donations). These problems have made the LT program in Indonesia less agile. At present, Indonesia has a National Transplant Committee under the Minister of Health in charge of carrying out the programs proposed above, but its implementation in the field of LT has not been optimal.
This study presents the novel characteristics of LLDs in Indonesia. Moreover, we recommend that the donor selection pathway of CMH become a national guideline or policy. A substantial LT campaign may help to improve the outcomes of LDLT in the future. More advanced diagnostic tools to measure the degree of fatty liver (magnetic resonance spectroscopy) and evaluate biliary anatomy (hepatobiliary phase of MRI using Gd-based contrast media) were not available in our center. We evaluated for fatty liver using a transient elastography (FibroScan, Echosens) and/or ultrasonography (routinely performed preoperatively) because we depended more in clinical examinations. However, we still encourage applying those technologies at our center to promote the possibility of laparoscopic hepatectomy for living donors.
In conclusion, LDLT in Indonesia has increased over the years, mainly in adult-to-pediatric LDLT. Our study showed a predominance of female donors, and the most frequently used graft type was the left lateral graft. The donors were often family related to the recipients. No donor mortality was found. However, there remains a scarcity of donors for adult-to-adult LT due to cultural differences and the challenge of identifying donors who meet medical selection criteria.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download