Abstract
Background
There are several procedural variations for kidney transplant donors, including open, laparoscopic, hand-assisted, and robotic methods, with either an intra-abdominal or retroperitoneal approach. Conversely, fewer options are available for the recipient procedure. We introduce a method that involves a small incision, with the goal of being less invasive for recipients.
Methods
Our current method was introduced in April 2022. As of July 2023, we have completed 27 cases. We analyzed several factors in these 27 cases, including the size of the incision, rewarming time, anastomosis time, graft function, analgesic use, and complications.
Results
The average incision size was 73 mm. The time taken for anastomosis was 24. 1 minutes, while the rewarming time averaged 43.1 minutes. There were no instances of primary nonfunction. One case necessitated postoperative dialysis three times due to heart failure. Following stent removal, one patient developed grade 1 hydronephrosis. There was one instance of bleeding from the drain insertion site. Another case involved a clamp injury to the external iliac artery, which necessitated stent insertion on the fourth postoperative day. Compared to procedures performed using conventional methods, the use of analgesics was less in these cases.
Generally, smaller incisions are viewed as less invasive and can contribute to a quicker recovery [1]. For a long time, the recipient procedure in kidney transplantation has remained largely unchanged, with only a few reports of the procedure being performed with a minimal skin incision [2]. Currently, other minimally invasive techniques, such as robotic procedures, are being explored [3]. Starting in April 2022, we altered kidney transplant recipient procedures from the traditional method to a technique involving a smaller incision. In this study, we analyzed the 27 cases in which we used this technique until July 2023, focusing on graft function, complications, and analgesic use.
This research did not involve any additional procedures beyond the original surgical procedure and regular clinic visits after discharge. The Institutional Review Board of Miyazaki Prefectural Hospital waived the need for approval for this particular study (waiver no. 23-30), and informed consent has been obtained.
We analyzed 27 consecutive kidney transplant recipients at Miyazaki Prefectural Hospital from April 2022 to July 2023. The analysis focused on graft function, analgesic use, incision size, rewarming time, anastomosis time, and complications
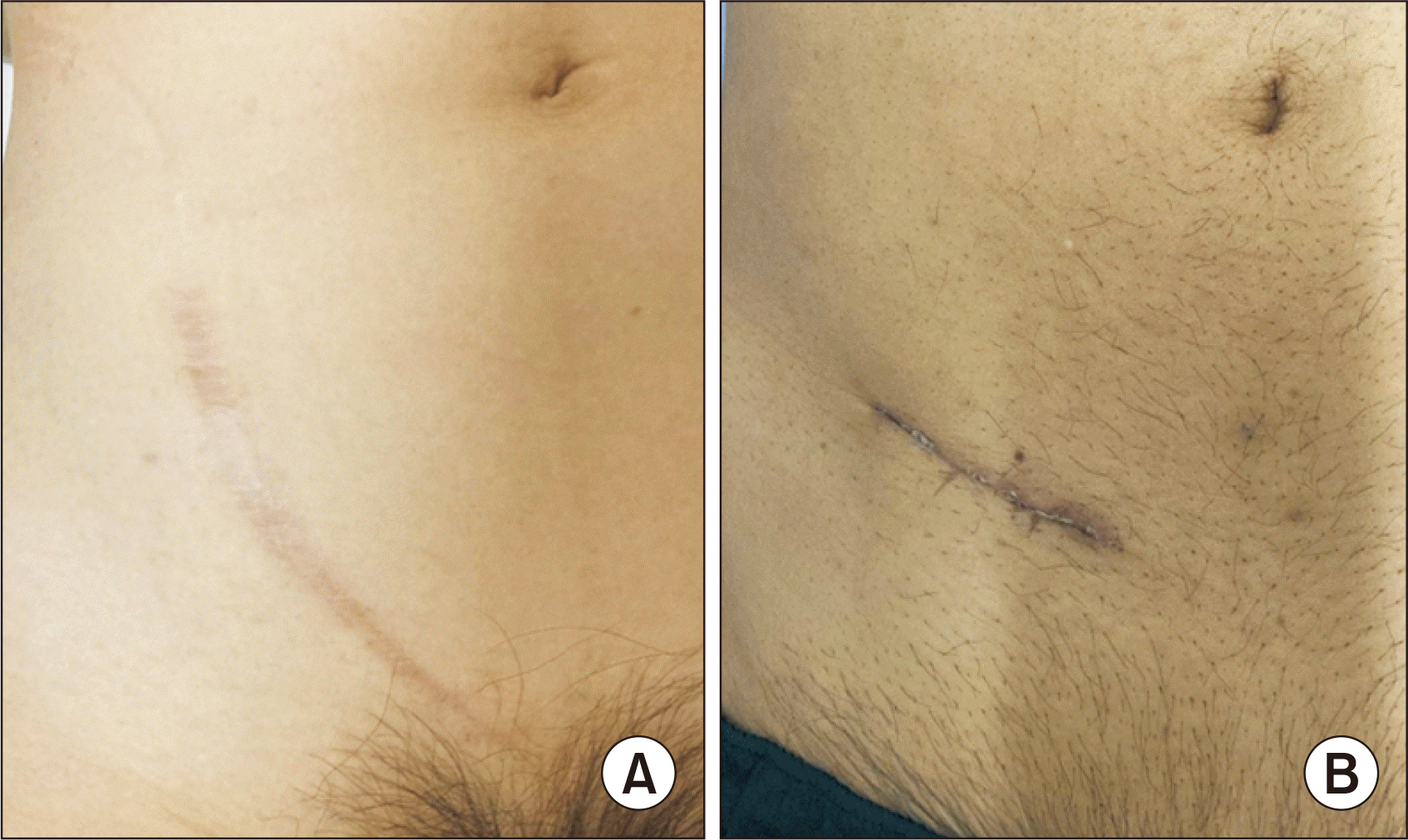
All procedures were performed under general anesthesia with the patient in a supine position. An incision of approximately 7 cm was made along the Langer's skin line (Fig. 1). The Retzius space was accessed below the arcuate line. The external oblique fascia, internal oblique fascia, and transabdominal fascia were dissected layer by layer at the attachment to the rectus muscle fascia. The peritoneum was reflected medially, and the external iliac artery and vein were exposed, securing space for the graft cranially. The graft was inserted through the incision prior to vessel anastomosis. Venous anastomosis was performed end-to-side with 6-0 Prolene. The posterior wall was sutured intraluminally, then over and over for the anterior wall. Arterial anastomosis was performed end-to-side using the parachute method with 6-0 Prolene. After reperfusion, the graft's color was assessed by direct vision by retracting the abdominal wall anteriorly, and the graft's blood flow was examined by ultrasound. The retractor's position was then changed to the direction of the bladder for ureterocystostomy. Ureterocystostomy was performed using the Lich-Gregoir technique with the ureteric stent inserted. The fascia was closed layer by layer with No. 1 polydioxanone suture (PDS), and the skin was closed with a subcutaneous running suture using 4-0 PDS.
The characteristics of the current 27 cases involving a small incision, as well as the most recent 27 cases using the conventional method conducted prior to the introduction of the small incision, are presented in Table 1. The results of the comparison between the two groups are displayed in Table 2. There were no instances of primary nonfunction, except for one case in the small incision group that required postoperative dialysis three times due to heart failure. All other cases achieved immediate graft function (Fig. 2). One case developed grade 1 hydronephrosis after stent removal and is currently still on a stent. One case experienced bleeding from the drain insertion site in the small incision group, and two cases were taken back to the operating room for hematoma evacuation. We administer fentanyl until extubation in the intensive care unit (ICU) on postoperative day 1, and provide acetaminophen as needed after extubation according to patient requests. The dosage of acetaminophen used in the small incision group was significantly less than the dosage used in the conventional group.
In conclusion, the procedure involving a small incision for kidney recipients proved feasible in terms of short-term graft function. The level of pain may be minor, as indicated by the analgesic use. The rate of complications was acceptable.
Kidney recipient procedures have long been performed with few variations [3]. Traditionally, hockey stick incisions or Gibson incisions of approximately 15 cm have been utilized. In our small incision method, the incision is made along the Langer's line of the skin, which results in less pain for patients. Additionally, the reduced dissection of the extraperitoneal space helps to minimize the risk of lymphocele [4] or bleeding. In the small incision group, there was one case where the patients were returned to the operating room to halt bleeding, and in the conventional group, there were two such cases. Both of these cases in the conventional group involved a hematoma that developed in the dissected retroperitoneal space. Conversely, one case in the small incision group experienced bleeding from the muscle at the drain insertion site. This patient had previously experienced subcutaneous bruises multiple times and was preoperatively prepared with plasma exchange and rituximab. The preoperative preparation, in conjunction with her potential primary hemostasis deficiency, may have caused the bleeding. Increased dissection space could heighten the risk of bleeding or lymphoma [4]. Furthermore, it appears reasonable that a smaller incision reduces the risk of developing an incisional hernia [5], although no patients in either group developed an incisional hernia in this series. Currently, some facilities are advocating for robotic recipient procedures [3]. These robotic procedures involve a small abdominal incision and several port placements. Moreover, the intra-abdominal approach used in the robotic procedure requires dissection of the peritoneum, and any contact or isolation of the bowel could potentially lead to postoperative ileus. Additionally, torsion of the renal graft is most commonly reported in grafts placed intra-abdominally, but it can also occur in some cases in the extraperitoneal space [6-8]. Therefore, even with the closure of the retroperitoneum at the end, the abdominal cavity approach in the robotic procedure could increase the risk of torsion over time. Our small incision method, which involves a minimal dissection of the retroperitoneal space, may be less invasive than the robotic procedure from this perspective.
Technically, it is crucial to sew the vessel with each donor and recipient side in intima-to-intima contact to prevent clot formation. The level of incision is also important to ensure optimal access to the vein and artery without any tension. Typically, the medial edge of the incision starts from three finger breadths above the pubic symphysis and one finger breadth lateral to the midline. Then, the incision follows the skin fold laterally for 6 to 7 cm, depending on the width of the graft size. However, if the graft size is large or the recipient's body mass index (BMI) is too low, the level of the incision should be higher. In such cases, we start the incision from four finger breadths from the pubic symphysis to place the graft more cranially in the retroperitoneal space. In one case, the right kidney was used as a graft from her husband, with a short thin renal vein, and the recipient's BMI was only 17.7 kg/m2. The vein was torn due to tension, and we closed the original anastomosis site and established a new anastomosis site more cranially. Our protocol is to continue administering fentanyl from the operation until extubation in the ICU. After extubation, acetaminophen is used as needed at the patient's request. The amount of acetaminophen used was less in the small incision group than in the conventional group. One issue that remains unresolved is that by placing the graft inside first, we cannot cool the graft during the anastomosis. The average rewarming time was 41.5 minutes, and during this time, we did not experience primary nonfunction or delayed graft function, except in one case of delayed graft function due to heart failure.
ARTICLE INFORMATION
Author Contributions
Conceptualization: YM. Data curation: MO. Formal analysis: YM. Investigation: YN. Methodology: YM. Project administration: YM. Visualization: YM. Validation: NYI. Writing–original draft: all authors. Writing–review & editing: YN. All authors read and approved the final manuscript.
REFERENCES
1. Wagenaar S, Nederhoed JH, Hoksbergen AW, Bonjer HJ, Wisselink W, van Ramshorst GH. 2017; Minimally invasive, laparoscopic, and robotic-assisted techniques versus open techniques for kidney transplant recipients: a systematic review. Eur Urol. 72:205–17. DOI: 10.1016/j.eururo.2017.02.020. PMID: 28262412.
2. Øyen O, Scholz T, Hartmann A, Pfeffer P. 2006; Minimally invasive kidney transplantation: the first experience. Transplant Proc. 38:2798–802. DOI: 10.1016/j.transproceed.2006.08.102. PMID: 17112833.
3. Spiers HV, Sharma V, Woywodt A, Sivaprakasam R, Augustine T. 2021; Robot-assisted kidney transplantation: an update. Clin Kidney J. 15:635–43. DOI: 10.1093/ckj/sfab214. PMID: 35371439. PMCID: PMC8967665.
4. Haberal M, Boyvat F, Akdur A, Kırnap M, Özçelik Ü, Yarbuğ Karakayalı F. 2016; Surgical complications after kidney transplantation. Exp Clin Transplant. 14:587–95.
5. Gioco R, Sanfilippo C, Veroux P, Corona D, Privitera F, Brolese A, et al. 2021; Abdominal wall complications after kidney transplantation: a clinical review. Clin Transplant. 35:e14506. DOI: 10.1111/ctr.14506. PMID: 34634148. PMCID: PMC9285099.
6. Ozmen MM, Bilgic I, Ziraman I, Koc M. 2013; Torsion of extraperitoneally transplanted kidney: an unusual complication. Exp Clin Transplant. 11:186–90. DOI: 10.6002/ect.2012.0089. PMID: 23075049.
7. Winter TC, Clarke AL, Campsen J. 2013; Acute torsion of a retroperitoneal renal transplant mimicking renal vein thrombosis. Ultrasound Q. 29:203–4. DOI: 10.1097/RUQ.0b013e31829d35cf. PMID: 23867571.
8. Lucewicz A, Isaacs A, Allen RD, Lam VW, Angelides S, Pleass HC. 2012; Torsion of intraperitoneal kidney transplant. ANZ J Surg. 82:299–302. DOI: 10.1111/j.1445-2197.2011.05792.x. PMID: 22507693.
Fig. 2
Serum creatinine (Cr) levels before and after kidney transplantation. (A) Small incision group. (B) Conventional group. Each color of the graph corresponds each patient.
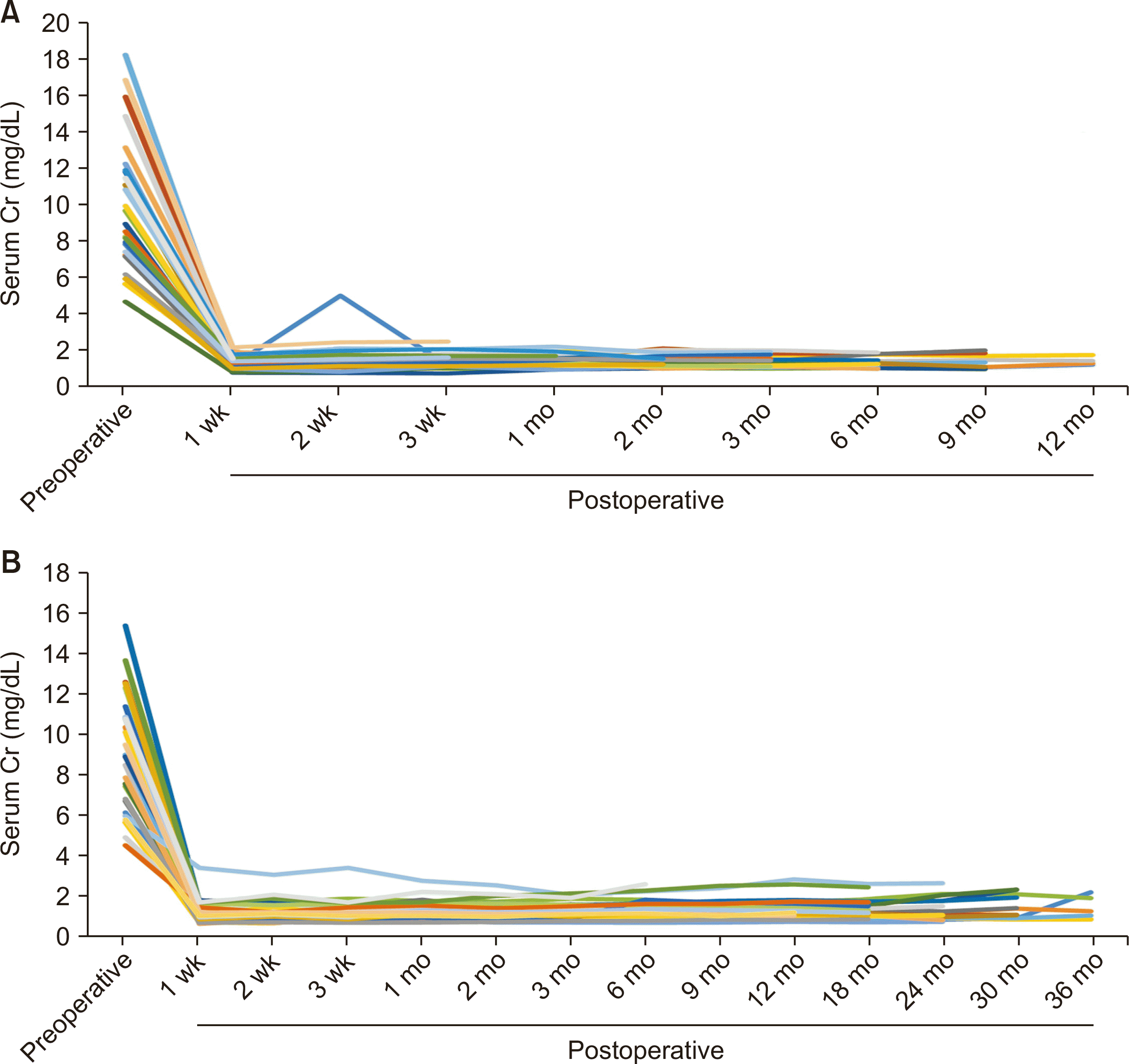
Table 1
Basic characteristics of small incision group and conventional incision group
Table 2
Comparison of results between the small-incision group and the conventional incision group
| Variable | Small incision (n=27) | Conventional (n=27) | P-valuea) |
|---|---|---|---|
| Incision size (mm) | 73 | 125 | <0.001 |
| Rewarming time (min) | 43.1 | 51.9 | 0.004 |
| Total operation time (min) | 183.2 | 189.4 | 0.577 |
| Anastomosis time (min) | 24.1 | 29 | 0.016 |
| Ureteroneocystostomy time (min) | 19.1 | 20.7 | 0.460 |
| Blood loss (mL) | 244.2 | 346.2 | 0.354 |
| No. of arteries | - | ||
| 3 | 2 | 0 | |
| 2 | 8 | 9 | |
| 1 | 17 | 18 | |
| Day of discharge (postoperative day) | 15.1 | 15.5 | 0.666 |
| Graft laterality (right:left) | 5:22 | 7:20 | - |
| Graft weight (g) | 180 | 162.8 | 0.180 |
| Fentanyl (mg) | 1.57 | 1.53 | 0.440 |
| Acetaminophen (g) | 1.07 | 2.81 | <0.001 |
| Complications | - | ||
| Bleeding | 1 | 2 | |
| Clamp injury | 1 | 0 | |
| Hydronephrosis | 1 | 0 | |
| Body mass index (kg/m2) | |||
| Donor | 23.5 | 23.2 | 0.710 |
| Recipient | 23.3 | 21.4 | 0.058 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download