Abstract
Background
Studies on the specificity of ABH antigen-antibody interactions at different pH values are rare. Therefore, the aim of this study was to estimate the effect of acidification of the reacting medium on the agglutinating ability of anti-A monoclonal antibodies (mABs) and their inhibition by glycoconjugates of red blood cell membranes.
Methods
Anti-A mABs were obtained from the Fourth International Workshop on Monoclonal Antibodies Against Human Red Blood Cell and Related Antigens (on July 19-20, 2002, in Paris, France). The glycoconjugates were isolated from erythrocytes' membranes. The inhibition of the lipid and protein isotypes of the blood group A antigen was assessed.
Results
The acidic medium decreased the agglutinating ability of acid immunoglobulin M (IgM) anti-A mABs, in contrast to alkaline immunoglobulin G (IgG) mABs. Meanwhile, at pH 6.5, IgM antibodies exhibited a high adsorption capacity, while IgG antibodies demonstrated a strong adsorption capacity at an alkaline pH. mABs 2-19, 2-28, 2-22, and 2-8 were inhibited by the acidic lipid and protein glycotopes of erythrocyte membranes. However, mAB 2-8 was inhibited by both acidic and alkaline glycotope variants.
The acidity of the medium has been demonstrated to influence the agglutination strength of red blood cells (RBCs) by polyclonal antibodies. Specifically, a donor's serum that contains only one of the two types of antibodies—either naturally occurring α agglutinins without immune anti-A antibodies or naturally occurring α agglutinins without immune anti-B antibodies—exhibits a diminished agglutinating capacity. It has been reported that anti-A or anti-B antibodies are absent in donors exhibiting the agglutination negative adsorption positive (ANAP) phenomenon, where agglutination is negative but adsorption with complement fixation is positive. This is observed in the case of the Ax blood group antigen, O(I) Ac'+, which indicates an O blood group type with the ability to adsorb anti-A antibodies [1].
Weakened agglutination of erythrocytes in an acidic medium by serum containing two types of antibodies was also observed when the donor's erythrocytes lacked the nonagglutinogenic blood group type A antigen isotype. This was revealed either by an adsorption reaction or by cell electrophoresis with complement (AB, Aс'–Bc'+, indicating the presence of anti-B antibody adsorbing ability and the absence of anti-A antibody adsorbing ability).
Reports of isoelectric differences in A, B, and H blood group glycotopes, which are associated with both lipids and proteins in the membrane of RBCs, have prompted studies into the isoelectric interactions of antigen-antibody interactions [2]. The variations in the isoionic points of glycotopes (pH 6.5–8.1) suggest differences in the charge of glycotopes at pH 7.4 [3,4]. Therefore, the change in glycotopes' charge in an acidic medium is a significant factor influencing the agglutinability of RBCs. This study sought to evaluate the influence of an acidic environment and the adsorption by lipid and protein glycotopes of erythrocyte membranes on the agglutination of RBCs by anti-A antibodies.
The study was conducted in accordance with the declaration of Helsinki. The protocol was approved by the Ethics Committee of Sytenko Institute of Spine and Joint Pathology, National Academy of Medical Sciences of Ukraine (No. 2). All persons gave their informed consent for participation in the study.
The erythrocytes and polyclonal sera were freshly drawn from 24 healthy volunteers aged 34.64±1.62 years old.
Monoclonal antibodies (mABs) were obtained through the program of the Fourth International Workshop on Monoclonal Antibodies Against Human Red Blood Cell and Related Antigens, which was dedicated to mABs against human RBC and related antigens. The workshop was organized under the auspices of the Institut National de la Transfusion Sanguine and the Etablissement Francais du Sang, and it took place on July 19-20, 2002 in Paris, France [5]. The mABs used in the study, including anti-A 2-17, anti-A 2-19, anti-A 2-22, anti-A 2-23, anti-A 2-8, anti-A 2-28, and anti-A BRIC-145, were obtained from the International Blood Group Reference Laboratory, (Gamma Biologicals Inc.), and Ortho Clinical Diagnostics Laboratory, United States. The Helix pomatia extract used in the study was generously provided by Professor Otto Procop from the Humboldt University of Berlin, Germany.
Glycosphingolipids were extracted from the lipids of RBC membranes using the chloroform-methanol method in the Immunology Laboratory of the Sytenko Institute of Spine and Joint Pathology [6]. Glycoproteins were identified through the treatment of RBCs with a 1% trypsin solution (Spofa), using a previously described method at pH 6.6 [6,7]. The purification of glycotopes was carried out on DEAE-cellulose (Reanal), with elution in a NaCl gradient and decreasing pH, as well as on DEAE-Sephadex A-25 (FandaChem). The fractions were assessed both serologically and photometrically, using a SF-26 spectrophotometer (Specord, Analytik Jena) at wavelengths of 205 and 280 nm.
The hemagglutination reaction, along with the inhibition of agglutination by RBCs and glycotopes from RBC membranes, was conducted in 96-well plates using blood group type O, A, and A2 RBCs [8]. A2 RBCs were tested through their reaction with polyclonal antibodies and anti-H mAB. Their absence of agglutination with anti-A 2-24 mAB was also tested. The score was determined based on Marsh's method after incubating with RBCs for 60 minutes (Fig. 1) [9].
The impact of medium acidification on the agglutinating ability of polyclonal antibodies was assessed at a pH range of 6.8–7.4, serving as the control assay for comparison with mABs. Specifically, polyclonal sera containing only α or β immunoglobulin M (IgM) antibodies demonstrated reduced agglutination in an acidic medium (at pH 6.8–7 compared to pH 7.2–7.4). This was in contrast to sera containing both α (IgM) and anti-A (immunoglobulin M [IgG]) or β and anti-B antibodies (Table 1). Consequently, sera from donors possessing only the adsorbing type of antigens showed a decrease in the expression of agglutination of test RBCs in an acidic medium at pH 6.8–7 compared to pH 7.2–7.4. However, sera from individuals with both adsorbing and agglutinating types of antigens did not show a decrease in the strength of agglutination in an acidic medium. No hemolysis of erythrocytes was observed in the acidic medium.
The investigation of acid-type anti-A mABs at various pH levels revealed a group of anti-A mABs (2-19, 2-10, 2-27, 2-28) with reduced agglutination activity in an acidic environment. Conversely, an alkaline-type group of anti-A mABs demonstrated increased agglutinating activity: 2-22 (1:32–1:64), 2-23 (1:16–1:32), 2-8 (1:256–1:512), and BRIC-145 (1:4–1:8). Meanwhile, BRIC-131 showed equal agglutination titers at pH 6.5 and 7.4. Interestingly, in an acidic medium, mAB 2-28 demonstrated a lack of adsorption ability with blood group type A2 RBCs, in contrast to its interaction with blood group type A1 RBCs (Table 2).
The agglutinating ability of anti-A mABs varied at pH 7.4 and 6.5, distinguished by high and low inhibition: an inhibition index >5 was observed for 2-28, 2-22, 2-19, and 2-17 mABs, while an inhibition index <5 was noted for mABs 2-8 and BRIC-145 mABs (Table 3). The group of mABs that exhibited a significant decrease in agglutination in an acidic medium contained IgM antibodies. Conversely, the group that demonstrated weak inhibition of agglutination contained IgG antibodies, specifically 2-8: IgG-3, 2-23: IgG-3, and BRIC-145: IgG-1.
Thus, the observed decrease in agglutination in an acidic medium by IgM α antibodies may be attributed to their diminished charge at pH 6.5. Conversely, the positive charge of alkaline IgG antibodies is hypothesized to increase. It has been reported that an acidic medium can enhance the fixation of incomplete agglutinins [7].
The analysis of the adsorption abilities of mABs demonstrated a stronger adsorption capacity at pH 6.5 compared to pH 7.4 for mABs 2-18, 2-23, 2-24, 2-19 (with blood group type A1 RBCs). Conversely, a stronger adsorption capacity at pH 8.2 compared to pH 6.8 was observed for mAB 2-23, BRIC-145, 2-8 (with blood group type A2 RBCs). Therefore, interesting differences at pH 6.5 were observed for mAB 2-28, which exhibited a decrease in agglutinating activity and an increase in adsorbing ability. Conversely, mAB 2-22 demonstrated an increase in agglutinating activity and a decrease in adsorbing ability.
The studied mABs differed not only in their enhanced adsorption capabilities at pH values of 6.5, 7.4, or 8.2, but also in their ability to adsorb various glycotopes of RBC antigens. mAB 2-8 adsorbed Alp-0, Apr-1, Alp-3, and Apr-3, as shown in Table 4. In contrast, mAB 2-19 adsorbed Alp-3 and Apr-3 (agglutination with blood group type A1 RBCs), but not Alp-0 and Apr-1. The adsorption capacity was stronger at pH 6.5 (1:1,024–1:256) than at pH 7.4 (1:1,024–1:512). mAB 2-28 demonstrated a stronger adsorption of Alp-3 and Apr-3 at pH 6.5 compared to pH 7.4 following contact with blood group type A1 RBCs. However, there was no observed inhibition of agglutination after contact with blood group type A2 RBCs. Conversely, mAB 2-22 demonstrated a higher adsorption activity at pH 7.4 compared to pH 6.5 for Alp-3 and Apr-3, and no adsorption was observed for Alp-0 and Apr-1. H. pomatia extract showed adsorption of Alp-3 and Apr-3. BRIC-131 adsorbed Alp-1.
The glycotopes of blood group type A have also been reported to exhibit differences in their isoelectric properties. These differences may influence agglutination, particularly in media with varying pH levels. The lipid glycotopes (Alp-00, Alp-0, and lp-3, with isoionic points at pH 8.1, 8, and 6.55, respectively) and protein glycotopes (Apr-1 and Apr-3, with isoionic points at pH 7.15 and 6.45, respectively) were utilized to investigate their inhibitory effects on anti-A antibodies [8]. The stability test results in most cases showed a coefficient of variation of <10%, indicating that reproducibility of the test.
When mABs were arranged based on the extent of reduction in their ability to be inhibited by acid protein glycoconjugates, the greatest decrease in the inhibition index was observed for mAB 2-28, while the smallest decrease was noted for mAB 2-8. Acid protein glycotopes are recognized as group A agglutinogens, specifically within blood group types A1, A2, and Ax3 RBCs. Interestingly, both protein and lipid acid glycoconjugates were able to inhibit the IgM 2-28 and 2-17 mABs, and both mABs exhibited a low level of inhibition by alkaline glycoconjugates (Alp-00). In an acidic medium, acid glycotopes exhibited a high inhibitory capacity for mABs 2-22 and 2-19. Intriguingly, glycoconjugates of protein origin inhibited the 2-22 mAB while glycoconjugates of lipid origin inhibited the 2-19 mAB. A marginal increase in inhibition by alkaline glycoconjugates was observed for mAB 222. For mAB 2-8 inhibition, the reaction with acid glycoconjugates was enhanced, while the agglutination with alkaline glycotopes was diminished.
The mABs studied demonstrated more potent agglutination at 4 °C than at 37 °C. There was a noticeable decrease in agglutination when the mABs were adsorbed. The agglutinating capacity of IgM mABs was diminished in an acidic medium. Therefore, not only may adsorption and elevated temperatures weaken the agglutinating ability of mABs, but the acidification of the medium may also have a similar effect.
Antibody-antigen interactions have been widely investigated [10,11]. Researchers have demonstrated that a high number of electrostatic interactions contributes to high binding specificity. Salt bridges and electrostatically controlled binding restrict flexibility due to geometric constraints. Conversely, hydrophobic binding and a low level of electrostatic interactions are associated with conformational flexibility. This results in antibodies having high specificity and affinity for antigens. The association between an antibody and an antigen involves noncovalent interactions. Amino acid residues on the antigen binding site, known as the epitope, and on the antibody binding site, known as the paratope, contribute to the stability of the antigen-antibody complex. Various methods used to study antigen-antibody complexes provide detailed visualizations of antigen-antibody interactions [11,12].
The pH of the medium has been reported to play a significant role in the formation of antigen-antibody electrostatic interactions. Antibodies that are positively charged (at pH 5–6), which promote repulsive self-interactions that contribute to high antibody stability, create robust non-specific interactions with negatively charged biomolecules at physiological pH. Conversely, antibodies with negatively charged Fv regions attract positively charged antigen molecules. Therefore, IgG1 antibodies with weakly basic isoelectric points (pI) ranging from 8–8.5, and Fv pIs between 7.5 and 9, exhibit the most effective combinations of strong repulsive self-interactions and weak nonspecific interactions [13].
The behavior of the solution is largely dependent on the charge status of the antibody at the desired pH [14]. Both the charge and its distribution are recognized as significant factors affecting protein solubility and solution viscosity [15,16]. It has been reported that the charge of the protein can influence its structure, stability, and its capacity to interact with other macromolecules [17,18]. After heating, polypeptides have been found to carry either a negative or positive charge at basic or acidic pH levels, respectively. An electrostatic repulsion force then acts to prevent the random entanglement of polypeptides, which is caused by a hydrophobic attractive force [19,20]. Subclasses of IgG significantly influence molecular properties, primarily due to their direct impact on the variable region. It has been reported that IgG1 mABs exhibit higher solubility than either IgG2 or IgG4 mABs at pH 6. Thus, the pH of the reacting medium for antigen-antibody binding occurs significantly impacts their interactions and warrants thorough investigation.
The study demonstrated the distinct impact of acidic pH on the agglutinating activity of mABs, which varied based on the class (IgM or IgG) and the isoelectric properties of the antibodies. Notably, a significant reduction in agglutinating activity was observed for IgM mABs in an acidic environment, in contrast to IgG mABs. Significant electrostatic interactions were identified between erythrocyte antigens and antibodies. Consequently, the agglutination reaction at pH values of 6.5, 7.4, and 8.2 facilitated the differentiation of two types of reactive mABs: those with increased agglutinating activity at pH 6.5 (2-22, 2-23, 2-8, BRIC-145) and those at pH 8.2 (2-28, 2-19, 2-17, 2-10, H. pomatia extract).
It is important to note that the mABs with distinct high agglutinating activity in an acidic medium also demonstrated strong adsorbing activity at pH 8.2, and vice versa. The 2-22, 2-23, and BRIC-145 mABs demonstrated strong agglutinating activity at pH 6.5 and high adsorption ability at pH 7.4. Conversely, the 2-28, 2-19, 2-17, and 2-10 mABs exhibited high agglutinating ability at pH 7.4 and strong adsorption ability at pH 6.5.
Interestingly, the mABs demonstrated varying abilities to react with A1 and A2 RBCs. Specifically, mAB 2-28 exhibited a strong reaction with blood group type A2 RBCs, as evidenced by a 1:8,192 titer at both pH 6.5 and 7.4. This contrasts with its reaction to blood group type A1 erythrocytes, which showed a 1:16 titer and a 1:2 titer at pH 6.5. However, mAB 2-28 did adsorb blood group type A1 RBCs, particularly at pH 7.4 (1:4–1:2), but did not adsorb A2 RBCs.
The adsorption capacity of mABs for A1 or A2 erythrocytes was determined by the pH of the medium. mAB 2-19 exhibits strong agglutination with blood group type A2 erythrocytes (at a 1:4,096 titer), in contrast to A1 RBCs, which show a lower titer of 1:16. Notably, mAB 2-19 demonstrates strong adsorption of A2 RBCs at pH 6.5, while at pH 7.4, there was increased adsorption of A1 RBCs. While mAB 2-24 demonstrated no agglutinating activity with blood group type A2 erythrocytes, the mABs under study exhibited varying abilities to adsorb glycotopes of both protein and lipid origin. These included Alp-3, Apr-3 (for 2-8, 2-19, 2-28, 2-22 mABs, as observed with H. pomatia extract), Apr-1 (2-18), and Alp-1 (BRIC-131). Thus, the reduction in antibody charge was primarily linked to a higher adsorption capacity rather than its agglutination ability. This is because for acidic mABs with a pI in an acidic medium, a decrease in agglutination and an increase in adsorption were observed in the same medium.
The use of an acidic medium helped to elucidate the differences in blood group type A antigens. In an acidic environment, the negative charge of RBCs diminishes, which results in more potent agglutination. The interaction of antibodies with varying isoelectric properties has identified two primary antigenic glycotopes: one acidic and the other alkaline, with both glycolipid and glycoprotein characteristics. The agglutinating ability of IgM mABs was diminished in an acidic medium compared to IgG mABs. The lack of reduced agglutination by IgG mABs, coupled with a high inhibitory index in an acidic medium (mABs 2-19 and 2-8), could be attributed to the increased charge of IgG mABs and their tropism towards the acidic nonagglutinogenic glycolipid antigens (Alp-3) [3], which retained their charge.
To summarize, the use of an acidic medium revealed specific interactions between alkaline glycotopes and acid α IgM agglutinins, as well as acid glycotopes with alkaline anti-A IgG antibodies. These interactions are not observed in individuals with low expression of acid complement-fixing glycolipid blood group type A antigens (Ac'– in AB Ac'–Bc'+ individuals who have anti-A antibodies but lack α antibodies [1]) or in individuals with type Ax (O Ac'+Bc'+ individuals who lack anti-A antibodies but have α antibodies [3]). The data obtained underscore the clinical significance due to the potential for hemolytic transfusion reactions. These reactions are relatively rare because of the presence of the immunosuppressive factor α- globulin in the serum of individuals with the ANAP phenomenon. This factor can block both the inductive and effector phases of the immune response, as well as complement activation [3].
The study did not investigate the agglutination inhibiting ability of the alkaline glycoprotein fractions associated with blood group type A glycoconjugates (Apr-00 and Apr-0). The data clearly demonstrated the significant influence of the charge of both glycoconjugates and antibodies on their mutual binding. It also highlighted the specificity of mABs towards blood group type A glycotope isotypes.
REFERENCES
1. Boyd WC. Fundamentals of immunology. 2nd ed. Interscience Publishers;1947.
2. Brockhaus M. Immunological research methods. Academic Press;1988. p. 160–2.
3. Delevsky YP, Gavrilova LV, Khavkina LV. 1980; Indicators of cellular immunity in its organ-specific manifestations in spinal osteochondrosis. Modern methods of treatment of degenerative-destructive diseases. Medicine. 1:S108111.
4. Delevskiĭ IP. Study of the nature of A1- and A2-antigenic differences with use of monoclonal antibodies: a role of glycoprotein and glycolipid epitopes in their formation. Klin Lab Diagn. 2006; (10):6–11. PMID: 17144537.
5. Proceedings of the Fourth International Workshop on Monoclonal Antibodies Against Human Red Blood Cell and Related Antigens. Under the Aegis of the Institut National de la Transfusion Saguine and of the Etablissement Francais du Sang. July 19-20. Paris, France. Transfus Clin Biol. 2002; 9:1–108. PMID: 11958157.
6. Conrath TB. Handbook of microtiter procedures. Dynatech Corp;1972.
7. Dausset J. 1953; Immunohematology of platelets and leukocytes. Presse Med (1893). 61:1533–5. PMID: 13134017.
8. Delevskiĭ IP, Zinchenko AA. Comparison of the antigenic spectrum A1, A2, and Ax erythrocytes: inhibition of mab with glucoconjugates of lipid and protein nature with different isoelectric properties. Klin Lab Diagn. 2007; (12):48–53. PMID: 18225514.
9. Marsh WL. 1972; Scoring of hemagglutination reactions. Transfusion. 12:352–3. DOI: 10.1111/j.1537-2995.1972.tb04459.x. PMID: 5072588.
10. Sinha N, Mohan S, Lipschultz CA, Smith-Gill SJ. 2002; Differences in electrostatic properties at antibody-antigen binding sites: implications for specificity and cross-reactivity. Biophys J. 83:2946–68. DOI: 10.1016/S0006-3495(02)75302-2. PMID: 12496069. PMCID: PMC1302377.
11. Sinha N, Li Y, Lipschultz CA, Smith-Gill SJ. 2007; Understanding antibody-antigen associations by molecular dynamics simulations: detection of important intra-and inter-molecular salt bridges. Cell Biochem Biophys. 47:361–75. DOI: 10.1007/s12013-007-0031-8. PMID: 17652781.
12. Kapingidza AB, Kowal K, Chruszcz M. 2020; Antigen-antibody complexes. Subcell Biochem. 94:465–97. DOI: 10.1007/978-3-030-41769-7_19. PMID: 32189312.
13. Yoshida K, Kuroda D, Kiyoshi M, Nakakido M, Nagatoishi S, Soga S, et al. 2019; Exploring designability of electrostatic complementarity at an antigen-antibody interface directed by mutagenesis, biophysical analysis, and molecular dynamics simulations. Sci Rep. 9:4482. DOI: 10.1038/s41598-019-40461-5. PMID: 30872635. PMCID: PMC6418251.
14. Gupta P, Makowski EK, Kumar S, Zhang Y, Scheer JM, Tessier PM. 2022; Antibodies with weakly basic isoelectric points minimize trade-offs between formulation and physiological colloidal properties. Mol Pharm. 19:775–87. DOI: 10.1021/acs.molpharmaceut.1c00373. PMID: 35108018. PMCID: PMC9350878.
15. Tang Y, Cain P, Anguiano V, Shih JJ, Chai Q, Feng Y. 2021; Impact of IgG subclass on molecular properties of monoclonal antibodies. MAbs. 13:1993768. DOI: 10.1080/19420862.2021.1993768. PMID: 34763607. PMCID: PMC8726687.
16. Raut AS, Kalonia DS. 2015; Opalescence in monoclonal antibody solutions and its correlation with intermolecular interactions in dilute and concentrated solutions. J Pharm Sci. 104:1263–74. DOI: 10.1002/jps.24326. PMID: 25556561.
17. Neergaard MS, Kalonia DS, Parshad H, Nielsen AD, Møller EH, van de Weert M. 2013; Viscosity of high concentration protein formulations of monoclonal antibodies of the IgG1 and IgG4 subclass - prediction of viscosity through protein-protein interaction measurements. Eur J Pharm Sci. 49:400–10. DOI: 10.1016/j.ejps.2013.04.019. PMID: 23624326.
18. Laue T. 2016; Charge matters. Biophys Rev. 8:287–9. DOI: 10.1007/s12551-016-0229-3. PMID: 28510017. PMCID: PMC5425809.
19. Liu S, Shah DK. 2023; Physiologically based pharmacokinetic modeling to characterize the effect of molecular charge on whole-body disposition of monoclonal antibodies. AAPS J. 25:48. DOI: 10.1208/s12248-023-00812-7. PMID: 37118220.
20. Emoto K, Yamashita S, Okada Y. 2005; Mechanisms of heat-induced antigen retrieval: does pH or ionic strength of the solution play a role for refolding antigens? J Histochem Cytochem. 53:1311–21. DOI: 10.1369/jhc.5C6627.2005. PMID: 16009962.
Fig. 1
(A) Anti-A 2-8 monoclonal antibody (mAB) with red blood cells (RBCs) with the blood group A antigen at 4 °C; 4+. (B) Anti-A mAB 2-8 with RBCs with the blood group A antigen at 37 °C; 3+. (C) mAB 2-19 with RBCs with the blood group A antigen at 37 °C at pH 6.0; 2+. (D) Anti-A mAB 2-23 with RBCs with the blood group A antigen at 37 °C; 1+. (E) Adsorbed anti-A mAB 2-8 with RBCs with the blood group A antigen at 37 °C; w+ (3 pt). (F) Adsorbed mAB 2-23 at 37 °C with RBCs with the blood group A antigen; w+ (1 pt) (A–F) ×400. pt, points.
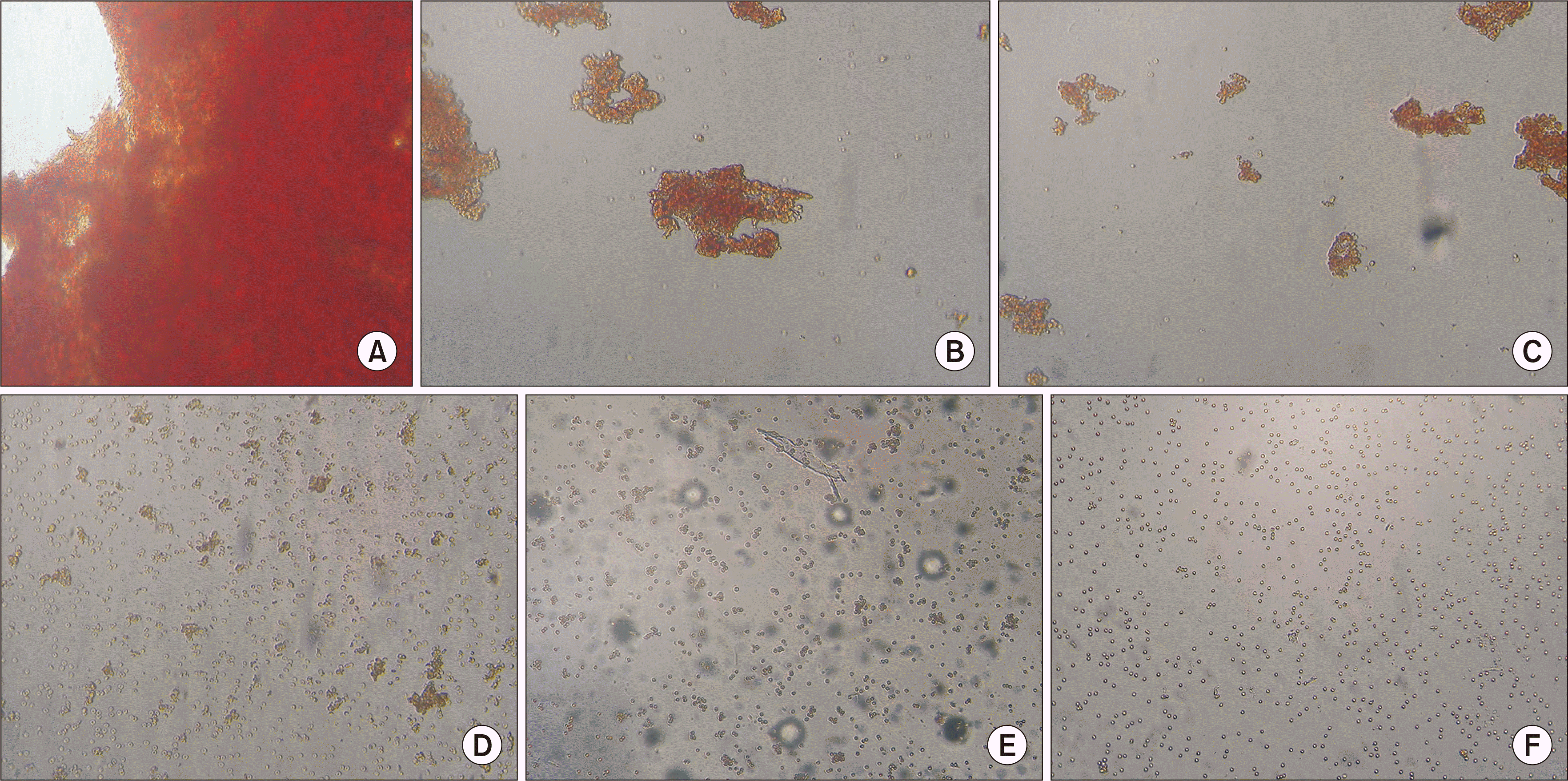
Table 1
The influence of an acidic medium on the agglutinating ability of polyclonal sera with complete and incomplete sets of antibodies
| Serum donor | Antibody | Erythrocyte | pH 6.8 | pH 7.0 | pH 7.2 | pH 7.4 |
|---|---|---|---|---|---|---|
| O Ac'+ | α | A | w+ | w+ | 1+ | 3+ |
| B Ac'+ | α | A | w+ | + | 3+ | 3+ |
| B Ac'– | α and anti-A | A | 3+ | 2+ | 3+ | 4+ |
| O Bc'– | β and anti-B | B | – | – | w+ | 3+ |
| A Bc'+ | β | B | – | + | 1+ | 4+ |
Table 2
Agglutinating and adsorbing abilities of mABs depending on the pH of the medium
Table 3
Adsorbing ability of mABs toward glycotopes with different isoelectric points




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download