Abstract
Background
Pretransplant therapies such as rituximab and plasmapheresis have led to an increase in ABO-incompatible (ABOi) living donor liver transplantation (LDLT), thus helping to overcome organ shortages. This study evaluated the changes in anti-A/B titers and CD19 levels over time in patients undergoing ABOi LT and aimed to understand the effect of single-nucleotide polymorphisms (SNPs) in Fc gamma receptor (FcγR) on rituximab therapy.
Methods
Two SNPs of FCGR2A (131H/R) and FCGR3A (158F/V) were identified. The clinical data on 44 patients who underwent ABOi LDLT between May 2019 and October 2021 at Seoul National University Hospital were reviewed retrospectively.
Results
Following desensitization with rituximab and subsequent LDLT, the anti-A/B titer recovered within 1 week, but decreased thereafter. The CD19 level increased at 3 months after LT. The genotyping data for FCGR3A (158F/V) indicated that two patients had the V/V genotype, and 42 had the F/V genotype. In the genotyping data for FCGR2A (131H/R), 21 patients had the H/H genotype, three had the R/R genotype, and 20 had the H/R genotype. However, there were no significant differences in anti-A/B and CD19 levels, bacteremia rates, T cell-mediated rejection, antibody-mediated rejection, or the survival rate among the FCGR2A types.
Conclusions
There were significant changes in the anti-A/B titers and CD19 levels over time in each patient after ABOi LDLT. The difference in outcomes following LT according to the FcγR SNP type for rituximab was unclear. Further studies with larger sample sizes are needed to confirm the effect of FcγR SNPs on rituximab therapy.
The increase in liver transplantation (LT) can be attributed to improved outcomes and the gradual increase in higher-risk transplants, including ABO-incompatible (ABOi) LTs and human leukocyte antigen-mismatching LTs [1-6]. Therefore, it is essential to understand the underlying immunological mechanisms in these high-risk transplants. Rituximab has been used to enhance the success of ABOi LT [6,7]. It is a monoclonal chimeric human-murine anti-CD20 immunoglobulin (Ig) G1 that eliminates B cells through the stimulation of complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and apoptosis [1,7]. The antibody-dependent cell-mediated cytotoxicity is initiated by the interaction between the Fc segment of immunoglobulin G (IgG) and the Fc gamma receptors (FcγRs) in monocytes, macrophages, dendritic cells, and natural killer cells. Therefore, allelic polymorphisms in these immune receptor cells potentially impact the effectiveness of rituximab [8]. Single-nucleotide substitutions in FCGR3A (158F/V; rs396991) and FCGR2A (131H/R; rs1801274) have been shown to influence the effectiveness of rituximab in lymphoma patients [9,10]. The V/V type (FCGR3A-158V homozygous) is associated with longer progression-free survival than the F/V type (FCGR3A-158F carrier) [9]. A higher incidence of urinary tract infections was found in kidney transplant recipients with the F/F type than in recipients with the V/V type [11]. Rejection-free survival and chronic allograft dysfunction following lung transplantation can differ depending on the FCGR2A and FCGR3A polymorphisms [12]. The V/V type showed more frequent acute rejection than the F/F or F/V type, and the R/R type had poorer chronic disease-free survival than the H/H or H/R types [12]. In LT, the occurrence of bloodstream infections differed significantly according to the type of FcγR polymorphism—specifically, the F/F or F/V types experienced more frequent bloodstream infections than the V/V type [13].
Few studies have investigated the influence of FcγR polymorphisms on rituximab effectiveness in ABOi LT, and those studies had relatively small sample sizes. Although genetic polymorphisms may vary by race, there are no studies specific to Korean patients undergoing LT. Therefore, we analyzed the characteristics and outcomes of ABOi LT with pretransplant rituximab treatment and plasmapheresis, as well as the effect of FcγR polymorphisms in the Korean population.
The study population included 44 patients, who received ABOi living donor LT (LDLT) between May 2019 and October 2021 at Seoul National University Hospital. This study was conducted according to the ethical guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 1904-046-1025). Informed consent was waived because of the retrospective nature of this study.
Our tailored desensitization protocol was previously described in detail [4,5], and is summarized in Fig. 1. Rituximab (300 mg/m2) was injected intravenously 2−3 weeks before LT, followed by one to six plasma exchanges (usually three) 1 week before LT, with a target ABO isoagglutinin titer <1:16. In titers >1:32, intravenous Ig (0.6−0.8 g/kg/day for 3−4 days postoperatively) with or without splenectomy was considered. Immunosuppression was achieved using a triple regimen including a steroid, tacrolimus (approximate target trough level 8−12 ng/mL for the first month, followed by 5−8 ng/mL thereafter), and mycophenolate mofetil, like that used for ABO-compatible LT. Basiliximab was excluded to avoid overdose of immunosuppressants.
Additional blood tests to assess ABO isoagglutinin titers and CD19 levels were conducted before rituximab administration: immediately before LT (PO), and postoperatively at 1 week (P1W), 1 month (P1M), 3 months (P3M), 6 months (P6M), 9 months (P9M), and 12 months (P12M), and then once or twice a year when necessary. A protocol biopsy was performed 1 week after LT. However, the biopsy was postponed or canceled when there was a high risk of bleeding or when the patient was relatively unstable.
Episodes of acute T cell-mediated rejection (TCMR) and antibody-mediated rejection (AMR) were determined based on biopsy-proven results. Bacteremia was defined as the detection of viable bacteria in a blood culture.
Genomic DNA was extracted from whole blood using a QIAamp DNA Blood Mini Kit (Qiagen) in accordance with the manufacturer’s instructions. The genomic region containing the polymorphism was amplified using 2X H-Star Taq PCR Pre-Mix (BioFACT). The polymerase chain reaction (PCR) cycle was as follows: 95 °C for 15 minutes, followed by 33 cycles of 95 °C for 30 seconds, 60 °C for 30 seconds, and 72 °C for 30 seconds. The following primers were used: (1) FCGR3A (158F/V; rs396991), 5’-TGTTGCTCCAGGCCCCTCGGT-3’ (forward) and 5’-TCCCAACTCAACTTCCCAGTG-3’ (reverse) and (2) FCGR2A (131H/R; rs1801274), 5’-ATCTTGGCAGACTCCCCATAC-3’ (forward) and 5’-TTTGTGTCTTTCAGAATGGCTGG-3’ (reverse). The PCR products (3 μL) were resolved on a 2% agarose gel and the amplicon size was compared with that of 1 Kb Plus DNA Ladder (BioFACT).
The study results were presented as numbers and mean values with percentages for categorical data and mean±standard deviation for continuous data. Statistical analyses were performed using IBM SPSS ver. 25 (IBM Corp.). Further statistical analyses were performed in consultation with a biostatistician working at the Medical Research Collaborating Center at Seoul National University Hospital. A mixed model was applied to data from repeated measurements of the same person. Four confounders (prerituximab immunoglobulin M [IgM] anti-A/B, number of plasmapheresis exchanges, TCMR or not, and AMR or not) were used as correction variables; the sample number was used as a random effect; and time, group, and the interaction thereof (time × group) was set as the fixed effect. In the mixed model, the mean values of IgM, IgG, and CD19 differed with the group (R/R or H/H vs. H/R) at each time point (PO, P1W, P1M, P3M, P6M, P9M, and P12M). If there was no significant difference, as observed through a nonsignificant interaction (time × group), the significance of time and group was evaluated after removing the interaction effect. In post-hoc analysis, type I errors due to multiple testings were corrected using the Bonferroni method. Analysis of covariance (ANCOVA) was performed to determine whether there was a difference in the mean values of IgM, IgG, and CD19 at each time point between the groups, using prerituximab IgM and the number of plasmapheresis exchanges as correction variables. P-values <0.05 indicated statistical significance.
The baseline characteristics and operative outcomes of all 44 patients are summarized in Table 1. The mean age and mean body mass index (BMI) were 56.9 years and 24.3 kg/m2, respectively. There were 29 patients (65.9%) with hepatocellular carcinoma. The mean Model for End-stage Liver Disease score was 10.9 and the operative time was 405.2 minutes. Three patients (6.8%) underwent simultaneous splenectomy. The mean IgM anti-A/B, IgG anti-A/B, and CD19 values before rituximab were 1:75.1, 1:319.1, and 16.4%, respectively, and the mean values before surgery were 1:2.6, 1:34.9, and 0.2%, respectively. The change in IgM and IgG titers and CD19 levels over time is shown in Fig. 2.
Genotyping data for FCGR3A (158F/V; rs396991) revealed that two patients (4.5%) were homozygous for FCGR3A (158V/V) and the other 42 (95.5%) were heterozygous with FCGR3A (158F/V). An additional set of genotyping data was analyzed for FCGR2A (131H/R; 1801274); 21 patients (47.7%) were homozygous for FCGR2A (131H/H), 3 (6.8%) were homozygous for FCGR2A (131R/R), and 20 (45.5%) were heterozygous for FCGR2A (131H/R). The number of patients who were homozygous for FCGR3A was small; therefore, this study focused on comparing the groups in FCGR2A (H/H vs. H/R or R/R) (Table 2).
All baseline characteristics were comparable between FCGR2A (131H/H) and FCGR2A (131H/R or R/R) except body weight (63.0 vs. 70.1 kg, P=0.034) and BMI (23.0 vs. 25.4 kg/m2, P=0.009). There were no significant differences in the operative findings, including the number of plasmapheresis exchanges or the rates of TCMR, AMR, and bacteremia between the two groups. The levels of IgM anti-A/B, IgG anti-A/B, and CD19 at each time point (prerituximab, PO, P1W, P1M, P3M, P6M, P9M, and P12M) were similar between the two groups.
The mean values of IgM anti-A/B, IgG anti-A/B, and CD19 were similar at each time point (PO, P1W, P1M, P3M, P6M, P9M, and P12M) between the two groups (P=0.499, P=0.999, and P=0.304, respectively) (Fig. 2). Analysis after excluding interaction (time × group) was performed. When adjusting for time, the mean IgM anti-A/B, IgG anti-A/B, and CD19 levels were all similar within each group (P=0.364, P=0.707, and P=0.256, respectively). When adjusting for group, the titer of IgM anti-A/B was similar between the two groups; however, there was a significant difference in the IgG anti-A/B titers and CD19 levels with time (P=0.002 and P<0.001). Specifically, there was a difference in the titer of IgG anti-A/B between P1W and PO (P=0.048), P1M (P=0.016), P3M (P=0.004), P6M (P=0.021), and P9M (P=0.049). There were differences in CD19 levels between P1W and P6M (P<0.001), P9M (P<0.001), and P12M (P<0.001); between P1M and P6M (P<0.001), P9M (P<0.001), and P12M (P<0.001); between P3M and P9M (P<0.001) and P12M (P=0.001); between P6M and PO (P<0.001); between P9M and PO (P<0.001); and between P12M and PO (P<0.001).
ANCOVA showed no differences in IgM anti-A/B, IgG anti-A/B, and CD19 levels between the two groups at different time points (P=0.984, P=0.522, and P=0.921, respectively). In addition, there were no significant differences in the overall survival rates and bacteremia-free survival rates between the two groups (Fig. 3).
ABOi LDLT is used widely for circumventing organ shortages, especially in Asia [1,5,14]. Many centers use plasma treatment procedures and immunosuppression strategies including rituximab administration to prevent hyperacute rejection, hepatic artery thrombosis, and immunological bile duct injury, which could result from a blood group antigen-antibody reaction in ABOi LDLT [1,5,8,15]. To evaluate and improve the outcomes of ABOi LDLT, it is important to thoroughly understand the effects and mechanisms of rituximab. Equally important is understanding how FcγR single nucleotide polymorphisms (SNPs) influence the effectiveness of rituximab. Moreover, it is imperative to address and minimize infections, which are frequent complications in ABOi LDLT and may be exacerbated by the additional immunosuppressive effects rituximab [1,6]. Notably, rituximab eliminates CD20+ cells through both complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxic mechanisms [1,6].
This study demonstrated real-world outcomes and the perioperative changes in anti-A/B titers and CD19 levels in ABOi LDLT. The ABOi LT protocol at our hospital is based on both rituximab and plasmapheresis. This study demonstrated that, with this protocol, anti-A/B IgM and IgG levels decreased considerably during LT, were restored within 1 week, and then decreased again. The recovery of antibody levels was consistent with results from previous studies [14,16]. The trends over time were similar for anti-A/B IgM and IgG. The CD19 levels decreased markedly during LT following rituximab and plasmapheresis; however, they started increasing 3 months after LT without a rebound at P1W. This indicates the effect of rituximab, which eliminates B cells, and its lasting effect based on the time of B cell reappearance. The increasing pattern in CD19 levels 3 months after LT is in accordance with a previous Japanese study [8].
To the best of our knowledge, this is the first study to identify SNPs in FCGR2A and FCGR3A in Korean patients undergoing LT. Unlike earlier studies, the majority of patients were heterozygous for FCGR3A (158F/V); only two patients had FCGR3A (158V/V) and none had FCGR3A (158F/F) [8,10,17]. This SNP bias limited our ability to study the differences in bacteremia rates among individuals with different SNPs. In other studies, the rate of bacteremia was highest in patients with the FCGR2A (131H/H) genotype and was higher in those with the FCGR3A (158F/V or F/F) genotypes than in those with the FCGR3A (158V/V) genotypes [8,17,18]. This study did not reveal any differences between the rate of bacteremia in patients with the FCGR2A (131H/H) genotype and the others. A comparison of individuals with FCGR3A SNPs was not possible because of bias in the number of individuals in each group. Therefore, further research is needed. However, if the findings of the previous studies are true and most Korean LT patients have the FCGR3A (158F/V) genotype, as shown in this study, Korean ABOi LT patients would be more susceptible to infections than those from other races.
A schematic graph demonstrating the trend of CD19 levels in this study shows lower CD19 levels 3 months after LT in patients with the FCGR2A (H/H) genotype than in those with the FCGR2A (H/R or R/R) genotype. However, unlike an earlier study, the difference was not statistically significant, possibly due to the small sample size [8]. Although this study did not show differences in the trend of anti-A/B IgM and IgG titers and CD19 levels between the two groups (FCGR2A [H/H] and FCGR2A [H/R or R/R]), it objectively showed significant differences in anti-A/B IgM and IgG titers and CD19 levels over time in each group.
This study had some limitations. First, it was a retrospective study and the available clinical information was dependent on the completeness of the electronic medical records. Second, there was a one-sided bias in one of the SNPs, which limited the comparison. Despite these limitations, this study demonstrated the real-world long-term trends of anti-A/B titer and CD19 levels over time in ABOi LT following rituximab treatment and plasmapheresis. In addition, this was the first study to identify SNPs in FCGR2A and FCGR3A in Korean patients undergoing LT.
In conclusion, there were significant changes in the anti-A/B titers and CD19 levels over time in all ABOi LT patients. Further genome-wide association studies using data from a large national database are needed to confirm the differences we found in the outcomes of ABOi LTs according to the FcγR SNPs. This could enhance the outcomes of ABOi LT through personalized patient-specific treatment approaches, including the adjustment of rituximab dosages and other immunosuppressive agents in accordance with the patient's specific polymorphism.
ARTICLE INFORMATION
Author Contributions
Conceptualization: SKH, KWL. Funding acquisition: SKH. Methodology: SKH, KWL, JML, YC, NJY, KSS. Data curation: SKH, JYK, JL, JK, HHC, SYH. Formal analysis: SKH, KWL. Writing–original draft: SKH, KWL. Writing–review & editing: all authors. All authors read and approved the final manuscript.
REFERENCES
1. Egawa H, Ohdan H, Saito K. 2023; Current status of ABO-incompatible liver transplantation. Transplantation. 107:313–25. DOI: 10.1097/TP.0000000000004250. PMID: 35849558. PMCID: PMC9875847.
2. Kok G, Verstegen MM, Houwen RH, Nieuwenhuis EE, Metselaar HJ, Polak WG, et al. 2022; Assessment of human leukocyte antigen matching algorithm PIRCHE-II on liver transplantation outcomes. Liver Transpl. 28:1356–66. DOI: 10.1002/lt.26431. PMID: 35152544. PMCID: PMC9544750.
3. Tajima T, Hata K, Kusakabe J, Miyauchi H, Yurugi K, Hishida R, et al. 2022; The impact of human leukocyte antigen mismatch on recipient outcomes in living-donor liver transplantation. Liver Transpl. 28:1588–602. DOI: 10.1002/lt.26511. PMID: 35603526. PMCID: PMC9796617.
4. Kim H, Yi NJ, Song EY, Lee K, Lee KW, Lee HW, et al. 2018; Preformed donor-specific antibodies do not affect the 1-year allograft survival in living donor liver transplantation. Clin Transplant. 32:e13244. DOI: 10.1111/ctr.13244. PMID: 29577436.
5. Natsuda K, Murokawa T, Lee KW, Yoon KC, Hong SK, Lee JM, et al. 2021; No diffuse intrahepatic biliary stricture after ABO-incompatible adult living donor liver transplantation using tailored rituximab-based desensitization protocol. Ann Transl Med. 9:30. DOI: 10.21037/atm-20-4703. PMID: 33553323. PMCID: PMC7859775.
6. Egawa H. 2020; Challenge to ABO blood type barrier in living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 19:342–8. DOI: 10.1016/j.hbpd.2020.06.017. PMID: 32665181.
7. Kessel A, Rosner I, Toubi E. 2008; Rituximab: beyond simple B cell depletion. Clin Rev Allergy Immunol. 34:74–9. DOI: 10.1007/s12016-008-8074-1. PMID: 18240027.
8. Sakai H, Tanaka Y, Tazawa H, Shimizu S, Verma S, Ohira M, et al. 2017; Effect of Fc-γ receptor polymorphism on rituximab-mediated B cell depletion in ABO-incompatible adult living donor liver transplantation. Transplant Direct. 3:e164. DOI: 10.1097/TXD.0000000000000683. PMID: 28620648. PMCID: PMC5464783.
9. Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. 2002; Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 99:754–8. DOI: 10.1182/blood.V99.3.754. PMID: 11806974.
10. Liu F, Ding H, Jin X, Ding N, Deng L, He Y, et al. 2014; FCGR3A 158V/F polymorphism and response to frontline R-CHOP therapy in diffuse large B-cell lymphoma. DNA Cell Biol. 33:616–23. DOI: 10.1089/dna.2013.2333. PMID: 25050883. PMCID: PMC4144364.
11. Das LK, Ide K, Tanaka A, Morimoto H, Shimizu S, Tanimine N, et al. 2017; Fc-gamma receptor 3A polymorphism predicts the incidence of urinary tract infection in kidney-transplant recipients. Hum Immunol. 78:357–62. DOI: 10.1016/j.humimm.2017.03.006. PMID: 28315348.
12. Paul P, Pedini P, Lyonnet L, Di Cristofaro J, Loundou A, Pelardy M, et al. 2019; FCGR3A and FCGR2A genotypes differentially impact allograft rejection and patients' survival after lung transplant. Front Immunol. 10:1208. DOI: 10.3389/fimmu.2019.01208. PMID: 31249568. PMCID: PMC6582937.
13. Shimizu S, Tanaka Y, Tazawa H, Verma S, Onoe T, Ishiyama K, et al. 2016; Fc-gamma receptor polymorphisms predispose patients to infectious complications after liver transplantation. Am J Transplant. 16:625–33. DOI: 10.1111/ajt.13492. PMID: 26517570.
14. Rummler S, Bauschke A, Baerthel E, Juette H, Maier K, Malessa C, et al. 2017; ABO-incompatible living donor liver transplantation in focus of antibody rebound. Transfus Med Hemother. 44:46–51. DOI: 10.1159/000450792. PMID: 28275333. PMCID: PMC5318927.
15. Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, et al. 2014; Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 61:575–82. DOI: 10.1016/j.jhep.2014.04.039. PMID: 24801413.
16. Ishida H, Kondo T, Shimizu T, Nozaki T, Tanabe K. 2015; Postoperative rebound of antiblood type antibodies and antibody-mediated rejection after ABO-incompatible living-related kidney transplantation. Transpl Int. 28:286–96. DOI: 10.1111/tri.12482. PMID: 25363583.
17. Hussain K, Hargreaves CE, Rowley TF, Sopp JM, Latham KV, Bhatta P, et al. 2019; Impact of human FcγR gene polymorphisms on IgG-triggered cytokine release: critical importance of cell assay format. Front Immunol. 10:390. DOI: 10.3389/fimmu.2019.00390. PMID: 30899264. PMCID: PMC6417454.
18. Sarmiento E, Cifrian J, Calahorra L, Bravo C, Lopez S, Laporta R, et al. 2018; Monitoring of early humoral immunity to identify lung recipients at risk for development of serious infections: a multicenter prospective study. J Heart Lung Transplant. 37:1001–12. DOI: 10.1016/j.healun.2018.04.001. PMID: 29754764.
Fig. 1
Desensitization protocol for ABO-incompatible living donor liver transplantation at Seoul National University Hospital. CMV, cytomegalovirus; LDLT, living donor liver transplantation; Bx, biopsy; POD, postoperative day; TAC, tacrolimus; MMF, mycophenolate mofetil; IVIG, intravenous immunoglobulin.
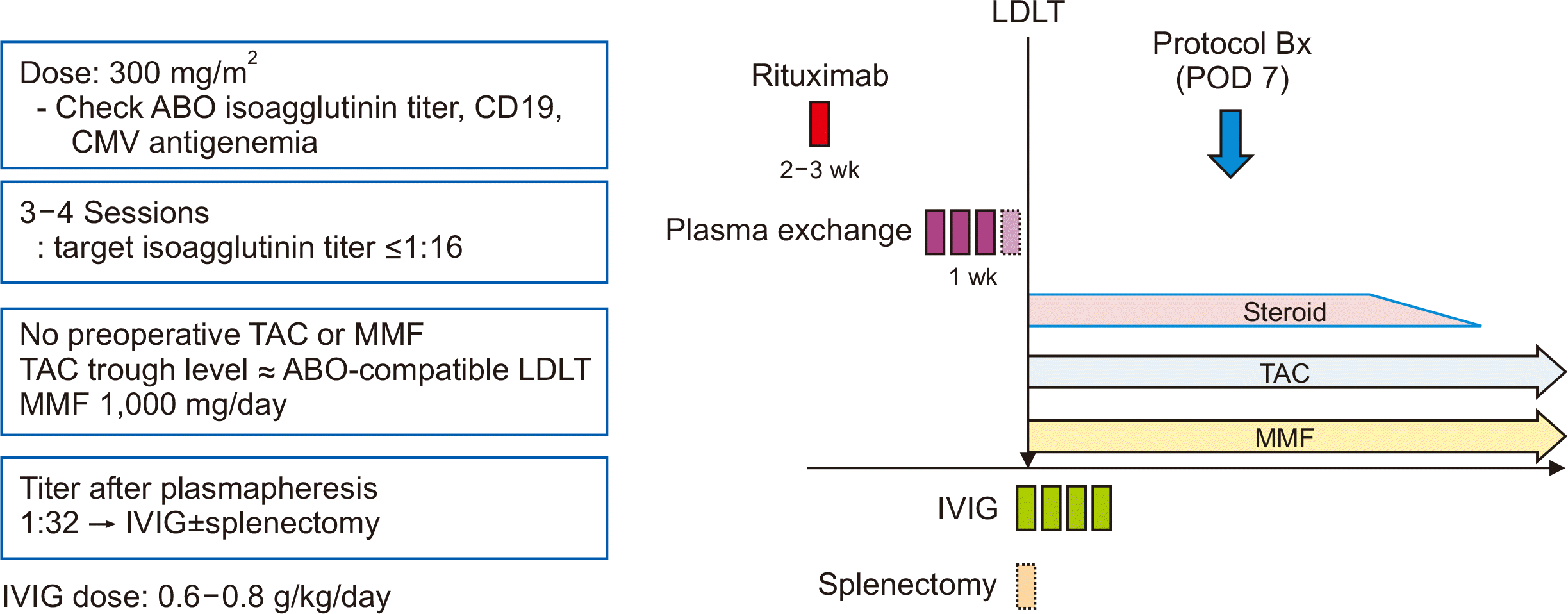
Fig. 2
Graph showing changes over time after ABO-incompatible living donor liver transplantation. (A) immunoglobulin M anti-A/B, (B) immunoglobulin G anti-A/B, and (C) CD19. PO, preoperative; P1W, postoperative week 1; P1M, postoperative month 1; P3M, postoperative month 3; P6M, postoperative month 6; P9M, postoperative month 9; P12M, postoperative month 12.
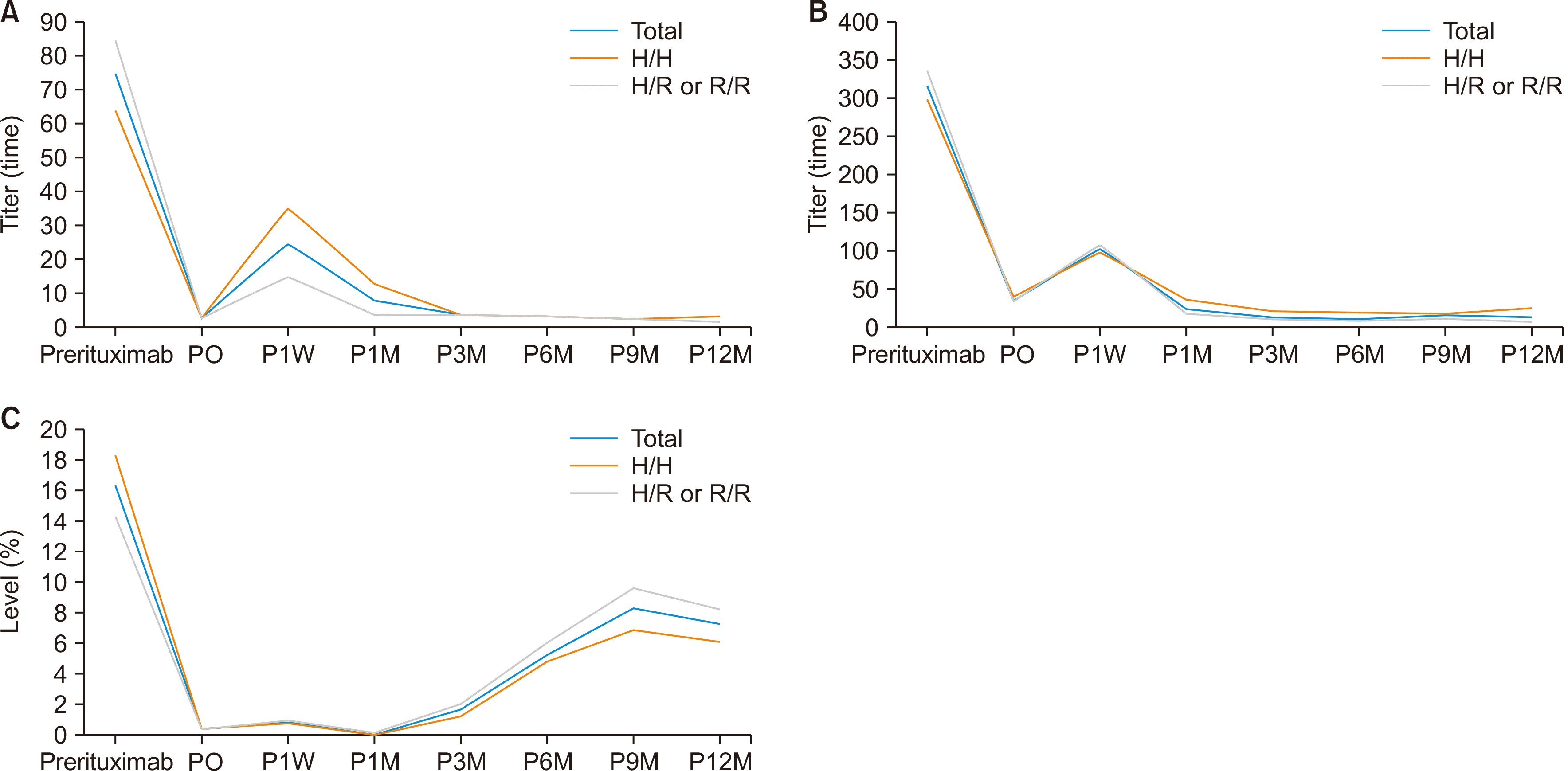
Fig. 3
Kaplan-Meier analysis of survival in patients who underwent ABO-incompatible living donor liver transplantation, comparing the FCGR2A (H/H) and FCGR2A (H/R or R/R) genotypes: (A) overall survival, (B) bacteremia-free survival. LT, liver transplantation.
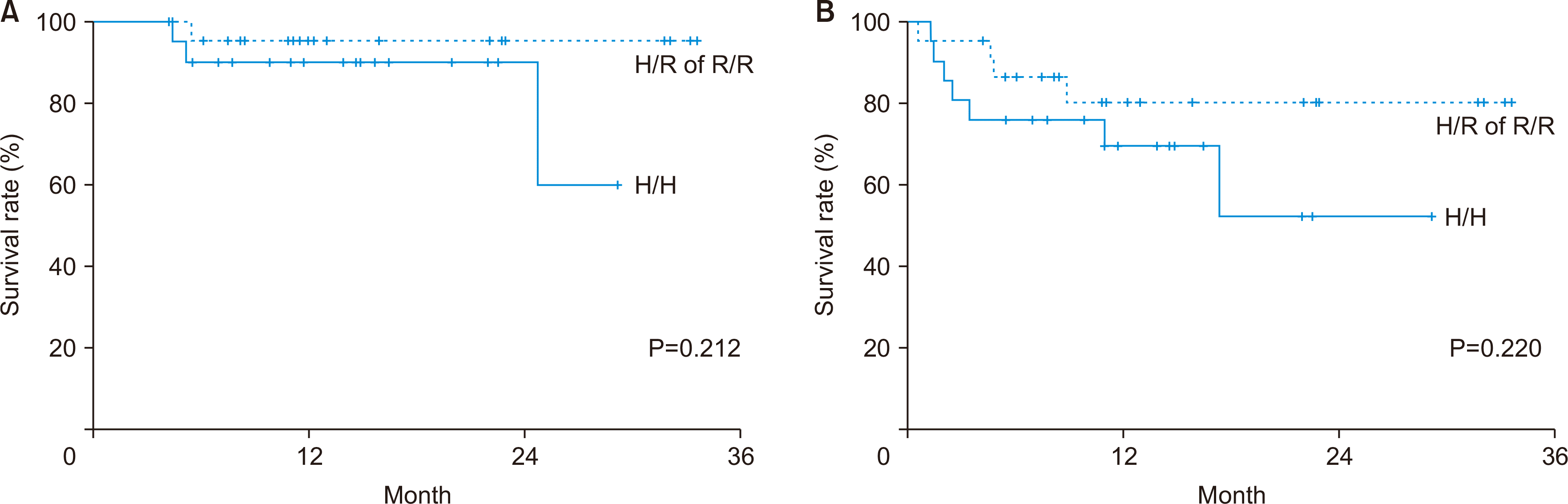
Table 1
Baseline characteristics and operative outcomes of 44 patients who received ABO-incompatible living donor liver transplantation after pretreatment with rituximab and plasmapheresis
Table 2
Comparison of FCGR2A (H/H) and FCGR2A (H/R or R/R) genotypes in patients who received ABO-incompatible living donor liver transplants




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download