INTRODUCTION
EVALUATION OF DECEASED DONORS FOR SARS-COV-2
Personal Protective Equipment for the Organ Procurement Team
Verification of the Medical History of the Deceased Donor
SARS-CoV-2 Testing for the Deceased Donor
Table 1
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; OPTN, Organ Procurement and Transplantation Network; TSANZ, The Transplantation Society of Australia and New Zealand; NHSBT, National Health Service Blood and Transplant; CNT, Centro Nazionale Trapianti (Italian Transplantation Society); CST, Canadian Society of Transplantation; LRT, lower respiratory tract; PCR, polymerase chain reaction; Ct, cycle threshold.
Modified from Peghin et al. [7] with permission of Elsevier according to the Creative Commons license.
Utilization of Cycle Threshold Values for SARS-CoV-2 PCR Testing in Deceased Donors
Utilization of SARS-CoV-2 Antibody Results in Deceased Donors
Utilization of Chest Computed Tomography for COVID-19
ORGAN PROCUREMENT FROM A DECEASED DONOR WITH A POSITIVE PCR TEST FOR SARS-COV-2
Organ Procurement and Transplantation of the Lungs and Small Bowel
Fig. 1
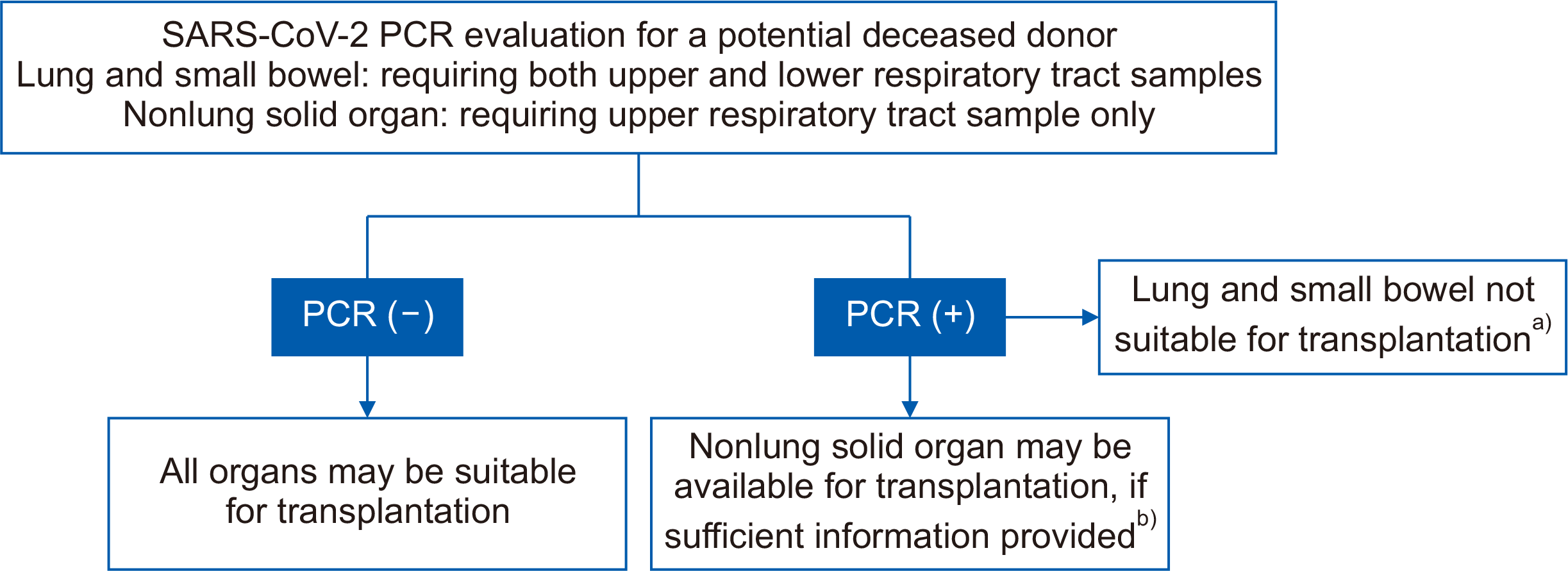
Table 2
| Study | Country | Study design | Study period | Donor definition and the number of donors | Number of recipients | Clinical outcome | Note |
|---|---|---|---|---|---|---|---|
| Goldman et al. (2023) [5] | United States | Retrospective cohort study from OPTN database | May 27, 2021–Jan 31, 2022 | 617 SARS-CoV-2 positive deceased donors (positive result within 21 days from the date of organ procurement) |
1,241 Recipients: 281 Kidney 106 Liver 36 Heart 11 Lunga) 14 Kidney-pancreas 5 Simultaneous liver-kidney 5 Simultaneous heart-kidney |
• Kidney: 30-day GF 0.8% (6/776), 30-day M 1.6% (2/776) • Liver: 30-day GF 3.8% (12/316), 30-day M 2.3% (10/316) • Heart: 30-day GF 3.8% (4/106), 30-day M 2.8% (3/106) • Lung: 30-day GF 0 % (0/11), 30-day M 0% (0/11) • Other: 30-day GF 3.1 % (1/32), 30-day M 3.1% (1/32) |
• No significant difference in 30-day graft/patient survival was observed between the graft outcomes from SARS-CoV-2-positive and -negative deceased donors. • No proven/probable DDTE case was reported. Only 1 possible case was reported (confirmed by DTAC). • DCD rate was higher in SARS-CoV-2-positive deceased donors. |
| Dhand et al. (2023) [23] | United States | Retrospective cohort study from OPTN database | Mar 2020–Dec 2021 | 193 SARS-CoV-2-positive deceased donors (positive result within 14 days from the date of organ procurement) |
414 Recipients (age ≥18 yr): 281 Kidney 106 Liver 36 Heart 1 Lung 14 Kidney-pancreas 5 Simultaneous liver-kidney 5 Simultaneous heart-kidney |
• Kidney: delayed graft function 21.8% (61/281), 30-day GF 0.7% (2/281), 30-day M 1.8% (5/281) • Liver: 30-day GF 4.7% (5/106), 30-day M 3.8% (4/106) • Heart: 30-day GF 0% (0/36), 30-day M 0% (0/36) • Kidney-pancreas: delayed graft function 14.3% (2/36), 30-day GF 7.1 % (1/36), 30-day M 0% (0/36) |
• No significant difference in 30-day graft/patient survival was observed between graft outcomes from SARS-CoV-2-positive and -negative deceased donors. • No COVID-19-related death was observed in the SARS-CoV-2-positive group. • The DCD rate was higher in SARS-CoV-2-positive deceased donors. |
| Free et al. (2022) [14] | United States | Retrospective cohort study from DTAC database | Mar 2020–Mar 2021 | 9 SARS-CoV-2-positive donors (positive result within 72 hours from the date of organ procurement, 6 living and 3 deceased donors) |
21 Organs and 19 recipients: 12 Kidney 4 Liver 3 Bilateral lungb) 1 Pancreas 1 Heart |
• 3 lung recipients (100%, 3/3) were confirmed DDTE, and 1 patient (33.3%, 1/3) died. | • None of the extrapulmonary organ recipients had evidence of DDTE. |
| Montiel Villalonga et al. (2023) [3] | Spain | Prospective case series study | Dec 15, 2020–May 31, 2022 | 32 SARS-CoV-2-positive deceased donors: 20, 10, and 2 SARS-CoV-2 positive, 0–14, 15–90, and >90 days after COVID-19 diagnosis |
69 Recipients: 41 Kidney 18 Liver 8 Heart 2 Simultaneous liver-kidney |
• 30-Day GF 5.8% (4/69, all failure from kidney recipients) • 30-Day M 2.9% (2/69, 1 from heart and 1 from liver-kidney recipient) |
• 4 Patients were posttransplant SARS-CoV-2-positive, but all patients had a history of close contact with confirmed COVID-19 patients. • No COVID-19-related death was observed. |
| Ushiro-Lumb et al. (2022) [4] | United Kingdom | Retrospective case series study | Mar 1, 2020–Mar 23, 2022 | 24 SARS-CoV-2-positive deceased donors (13 had a result profile suggesting previously resolved infection, 9 had indeterminate results, and 2 had results compatible with current asymptomatic infection) |
66 Organs and 64 recipients: 35 Kidney 19 Liver 3 Heart 3 Bilateral lung 4 Simultaneous pancreas-kidney |
NA | • Only 1 recipient (bilateral lung) was positive for SARS-CoV-2 by PCR 5 days after transplantation, but the evidence does not support the donor-derived transmission. |
| Romagnoli et al. (2021) [24] | Italy | Retrospective case series study | Nov 20, 2020–Feb 8, 2021 | 10 SARS-CoV-2-positive deceased donors (time between the first detection of SARS-CoV-2 positivity and organ procurement: 0–10 days) | 10 Liver recipients | • 1 Patient died 75 days after transplantation. | • No definitive COVID-19 transmission was observed. |
OPTN, Organ Procurement and Transplantation Network; SARS-C0V-2, severe acute respiratory syndrome coronavirus 2; GF, graft failure; M, mortality; DDTE, donor-derived transmission events; DTAC, Disease Transmission Advisory Committee; DCD, donation after cardiac death; COVID-19, coronavirus disease 2019; NA, not available; PCR, polymerase chain reaction.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download