Abstract
Bilateral distal anterior cerebral artery (DACA) aneurysms also called “kissing aneurysms” or “mirror aneurysm” are extremely rare, accounting for only 0.2% of all intracranial aneurysms. There have only been a few examples of mirror DACA aneurysms reported in the literature. Here, we report a rare case of mirror DACA aneurysm in a middle aged female with its successful clipping. Patient was admitted with severe headache and altered sensorium. Computed tomography (CT) head was suggestive of anterior inter-hemispheric hematoma. Digital subtraction angiography (DSA) was done which was suggestive of two distal anterior cerebral artery aneurysms located at same anatomical position. It was treated through microsurgical clipping. Mirror image DACA aneurysms are rare occurrence. All patients with ruptured DACA aneurysms should have angiography with 3D reconstruction studies. This aids in determining the aneurysm’s morphology and planning treatment accordingly.
Intracranial aneurysm is a relatively common vascular condition, with an incidence of 0.9 to 10% in the general population [10]. Aneurysms in the distal anterior cerebral artery (DACA) accounts for 4.4% of all intracranial aneurysms [5,26]. DACA aneurysms occur in the A2-A5 segments of the anterior cerebral artery (ACA) and its distant branches. Bilateral DACA aneurysms also called “kissing aneurysms” or “mirror aneurysms,” are even unusual, accounting for about 0.2% of all intracranial aneurysms [2], with just a few occurrences described to date. Jefferson [11] first described and defined “kissing aneurysms” in 1978, meaning two aneurysms in anatomically similar location that arise from two different arteries with separate origin and are in near vicinity with or without partially adherent walls. Management of such aneurysms is a challenging task due to difficult access to the region as the operative field for dissection is very narrow, the presence of dense inter-hemispheric adhesions, and the difficulty of distinguishing two different aneurysms due to partially adherent walls, the presence of important neurovascular structures and associated hematoma. We present a case of subarachnoid hemorrhage with anterior inter-hemispheric hematoma caused by rupture of bilateral DACA aneurysms, as well as its effective management.
A 50-year-old female reported in our institute with chief complaints of severe headache associated with episodes of vomiting and altered sensorium for the past one week. On examination, the patient’s glasgow coma score (GCS) was E3V3M5 and Hunt and Hess grade III. Vitals were stable after initial resuscitation. The patient had a past medical history of hypertension for which she was taking the anti-hypertensive medication without regular follow-up. Plain computed tomography (CT) head was done suggestive of anterior inter-hemispheric hematoma (Fig. 1A). CT angiography (CTA) brain revealed aneurysms in the bilateral A3 segment of ACA at the bifurcation of DACA into pericallosal and callosomarginal arteries (Fig. 1D). Digital subtraction angiography (DSA) of the brain of the patient was done to assess the anatomy and morphology of the aneurysms and plan intervention accordingly. DSA brain showed two aneurysms at the junction of pericallosal and callosomarginal arteries (Fig. 1B, C and Fig. 2A-D). The right DACA aneurysm was 2.3 mm×2.5 mm in size with a neck of 1.8 mm and the left DACA aneurysm was 1.9 mm×2.4 mm with a neck of 1.9 mm. Both of the aneurysms were directing antero-superiorly.
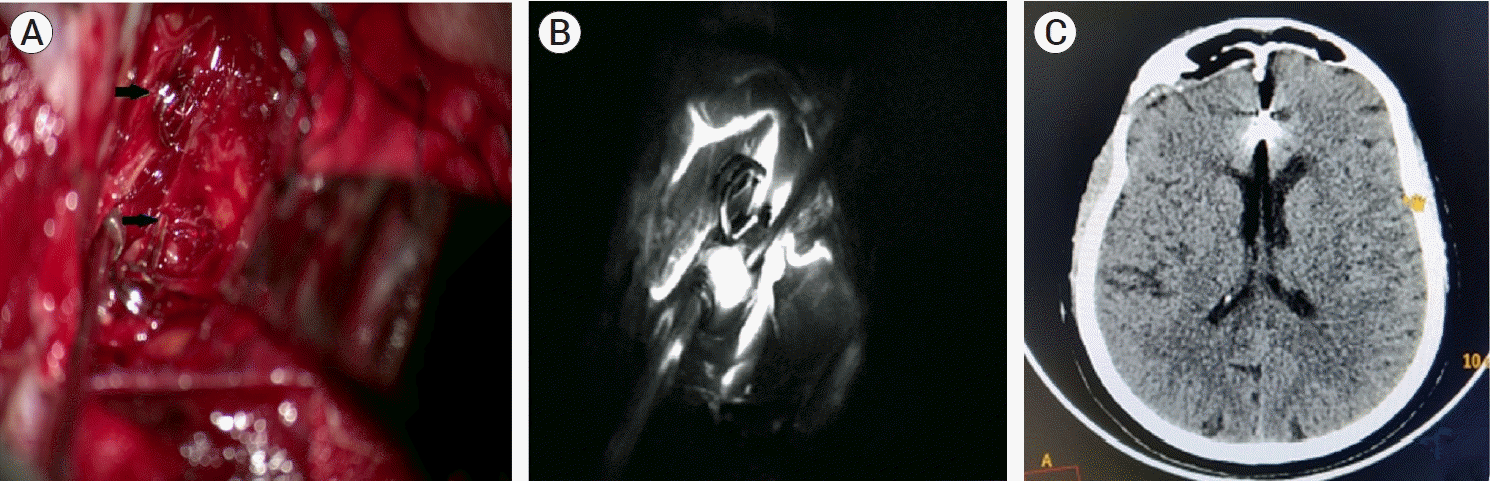
Endovascular coiling of both DACA aneurysms was planned. After the deployment of a coil in the left DACA aneurysm, the coil migrated into the parent artery during detachment Attempts were made to retrieve the dislodged coil were unsuccessful. Finally, we pushed the coil distally in one of the terminal branch of parent artery and posted the patient for microsurgical clipping after immediate reversal of heparinization with protamine. A bi-coronal skin flap was raised with a right frontal craniotomy extending 1 cm across the midline towards the left. Meticulous inter-hemispheric dissection was done in the midline between bilateral basifrontal lobes. Inter-hemispheric cisterns were opened and both aneurysms were located and proximal control was taken with temporary clips. The hematoma was evacuated and bilateral DACA aneurysms were clipped successfully with 5 mm clips each (Fig. 3A). Post-clipping, the blood flow in the parent artery and its branches were assessed with indocyanine green video angiography (ICG-VA) (Fig. 3B).
The postoperative period was uneventful and the patient was discharged on postoperative day eight with significant improvement and no neurological deficit (Modified Rankin Scale score - 2). Plain CT head at one month showed resolving hematoma with no evidence of any infarct (Fig. 3C).
Bilateral DACA aneurysms also called “Kissing Aneurysms” or “Mirror Aneurysms” are extremely rare, accounting for only 0.2% of all intracranial aneurysms [11]. There have only been a few examples of mirror DACA aneurysms reported in the literature. Komiya et al. found 0.9% of “kissing” aneurysms in their study [13], while Yasargil et al. and Sun et al. found 0.2% [24,27]. It was our institute’s first report of such a case.
The majority of these aneurysms form at the bifurcation of the ACA into the callosomarginal and pericallosal arteries in the A3 portion of the ACA around the genu of the corpus callosum. Harada et al. divided kissing aneurysms into two categories based on the position of the aneurysmal neck [7]. In type 1, each aneurysmal neck is positioned on the same parent artery, but in type 2, each aneurysmal neck is located on different parent arteries. In our case, the type 2 variant was present at the bifurcation. Congenital anomalies such as Marfan’s syndrome, Ehler’s-Danlos syndrome, and autosomal dominant polycystic kidney disease are all known to be frequently associated with bilateral DACA aneurysms [3]. Also anatomic variations in distal ACA are well known to occur. The flow disturbances and hemodynamic stress caused by such alterations in vascular structures might also account for mirror aneurysms [14]. Our case is an example of a rare occurrence in which a mirror DACA aneurysm was not associated with any congenital or vascular anomaly.
The majority of DACA aneurysms rupture while they are small and before any mass effect appears. Aneurysms less than 5 mm in diameter are more prone to rupture and cause subarachnoid hemorrhage or intracerebral hematoma [4,6,12,17,21]. In one series, 67% of patients of ruptured DACA aneurysms had aneurysms of less than 5 mm [18]. It was less than 3 mm in our case, which is itself a risk factor for spontaneous rupture. Inter-hemispheric and corpus-callosal hematoma is the most common presentation of ruptured DACA aneurysms. The incidence of intracerebral hematoma (ICH) in ruptured DACA aneurysms has been found to range between 40% and 70%. Ohno et al. and Sekerci et al. found that ICH occurs in 40% and 70% of ruptured DACA aneurysms, respectively [18,20].
CTA or digital subtraction angiography (DSA) brain is essential in cerebral aneurysms to determine the location, morphology, and number of aneurysms. CTA is very sensitive and specific for detecting cerebral vascular abnormalities but small aneurysms (<3 mm) can be missed in CTA [1]. Therefore, in clinical practice, DSA remains the gold standard technique for detecting vascular anomalies and both small and large intracranial aneurysms. It provides complete study of cerebral vasculature in real time and assists surgeons in determining if an endovascular or microsurgical operation is required for a given patient. In our situation, endovascular coiling appeared to be a viable option, but complications required surgical clipping. This further highlights the importance of dual training for neurosurgery residents in both endovascular and microsurgical procedures.
Endovascular therapy of distal ACA aneurysms is a significant challenge for neurosurgeons. The smaller size of aneurysms with a broad neck and a limited lumen of the parent artery presents a technical difficulty for endovascular treatment. Earlier case series of endovascular management of DACA aneurysms revealed lower success rates, however with current breakthroughs and refinements in endovascular therapy, we have witnessed improvements in success rates and outcomes. In their institutional investigation, Huang et al. reported a technical success rate of 97.6% and an aneurysm occlusion rate of 90.3% [9]. Suzuki et al. and Sturiate et al. reported a technical success rate of 66.3 percent and 78 percent, respectively, at the end of the coiling procedure for DACA aneurysm [22,25]. Endovascular therapy of mirror DACA aneurysms is difficult. Bilateral ACA must be cannulated individually, and because of the tiny lumen of the distal ACA, controlling and guiding the micro-catheter is extremely challenging. Because DACA aneurysms are smaller in size, issues such as coil compaction and coil migration must be considered. When compared to other aneurysms, coiling of DACA aneurysms is associated with much more difficulties [15].
Despite advances in endovascular therapy, microsurgical clipping can be considered as a treatment option in DACA aneurysms. Surgical clipping of DACA aneurysms may present with various difficulties, including a small working area in the interhemispheric fissure and the callosal cistern, a broad-based or sclerotic aneurysm neck, difficulties localizing the parent artery, and adherent walls of the mirror aneurysms. Another issue with these aneurysms is that they are more likely to rupture intraoperatively during exposure since the dome of the aneurysm generally projects towards the surgeon and is seen before the neck. A study by Suh et al. [23] reported an intra-operative rupture rate of 50% in DACA aneurysms surgery. Furthermore, because these aneurysms are frequently embedded in the frontal lobes, vigorous manipulation or traction may result in avulsion and rupture of these aneurysms.
An anterior inter-hemispheric approach with unilateral frontal craniotomy is mostly used to treat distal ACA aneurysms [28]. The pterional and sub-frontal approaches are also used, but these are preferred for aneurysms located in the proximal part of the ACA. The inter-hemispheric approach can be used to treat distal ACA aneurysms located anywhere other than the anterior communicating artery. Despite these obstacles, surgical procedures allow for the hematoma to be evacuated, reducing the mass effect and the duration and severity of vasospasm [8,16]. With clipping, the rate of aneurysm occlusion is also very high. In a study, Pandey et al. [19] discovered that patients who had DACA aneurysms clipped had a better outcome than the endovascular group. Despite the challenging features, microsurgical clipping remains the primary treatment modality for DACA aneurysms.
Mirror aneurysms in bilateral DACA are quite unusual. The exact identification of the ruptured aneurysm site in a patient with mirror DACA aneurysms is of utmost importance in order to initiate appropriate treatment and achieve safe aneurysm occlusion. Before the treatment, all patients with ruptured DACA aneurysms should have angiography with 3D reconstruction studies. This aids in determining the aneurysm’s morphology and planning treatment accordingly. Our reported case confronted us with several difficulties in deciding the course of treatment. Despite the anatomical challenges of the narrow corridor, microsurgical clipping of aneurysms can be a safer and successful treatment option in patients of ruptured mirror DACA aneurysms presenting with intracerebral hemorrhage.
REFERENCES
1. Alimohammadi M, Bidabadi MS, Amirjamshidi A. Bilateral “kissing” aneurysms of the distal pericallosal arteries: report of a case and review of the literature. Neurosurg Q. 2010; 20(4):308–10.
2. Baldawa SS, Menon G, Nair S. Kissing anterior communicating artery aneurysms: diagnostic dilemma and management issues. J Postgrad Med. 2011; Jan-Mar. 57(1):44–7.
3. Baptista AG. Studies on the arteries of the brain. II. The anterior cerebral artery: some anatomic features and their clinical implications. Neurology. 1963; Oct. 13:825–35.
4. Beck J, Rohde S, Berkefeld J, Seifert V, Raabe A. Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg Neurol. 2006; Jan. 65(1):18–25. discussion 25-7.
5. Choque-Velasquez J, Hernesniemi J. One burr-hole craniotomy: anterior inerhemispheric approach in Helsinki neurosurgery. Surg Neurol Int. 2018; Jul. 9:141.
6. Forget TR, Benitez R, Veznedaroglu E, Sharan A, Mitchell W, Silva M, et al. A review of size and location of ruptured intracranial aneurysms. Neurosurgery. 2001; Dec. 49(6):1322–5. discussion 1325-6.
7. Harada K, Orita T, Ueda Y. Large kissing aneurysms of the middle cerebral artery: a case report–classification of kissing aneurysms. No Shinkei Geka. 2004; May. 32(5):513–7.
8. Hernesniemi J, Tapaninaho A, Vapalahti M, Niskanen M, Kari A, Luukkonen M. Saccular aneurysms of the distal anterior cerebral artery and its branches. Neurosurgery. 1992; Dec. 31(6):994–8. discussion 998-9.
9. Huang Q, Shen J, Xu Y, Liu J. Endovascular treatment of ruptured distal anterior cerebral artery aneurysm. Neurol India. 2010; Mar-Apr. 58(2):259–63.
10. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms - risk of rupture and risks of surgical intervention. N Engl J Med. 1998; Dec. 339(24):1725–33.
11. Jefferson A. The significance for diagnosis and for surgical technique of multiple aneurysms of the same internal carotid artery. Acta Neurochir (Wien). 1978; 41(1-3):23–37.
12. Juvela S, Porras M, Heiskanen O. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J Neurosurg. 1993; Aug. 79(2):174–82.
13. Komiyama M, Yasui T, Tamura K, Nagata Y, Fu Y, Yagura H. “Kissing aneurysms” of the internal carotid artery. Neurol Med Chir (Tokyo). 1994; Jun. 34(6):360–4.
14. Kozyrev D, Jahromi B, Thiarawat P, Choque-Velasquez J, Ludtka C, Goehre F, et al. Three distal anterior cerebral artery aneurysms in the same branch associated with five additional intracranial aneurysms. Surg Neurol Int. 2017; Apr. 8:62.
15. Lehecka M, Dashti R, Lehto H, Kivisaari R, Niemelä M, Hernesniemi J. Distal anterior cerebral artery aneurysms. Acta Neurochir Suppl. 2010; 107:15–26.
16. Leheka M, Lehto H, Niemela M, Juvela S, Dashti R, Koivisto T, et al. Distal anterior cerebral artery aneurysms: treatment and outcome analysis of 501 patients. Neurosurgery. 2008; Mar. 62(3):590–601. discussion 590-601.
17. Ohashi Y, Horikoshi T, Sugita M, Yagishita T, Nukui H. Size of cerebral aneurysms and related factors in patients with subarachnoid hemorrhage. Surg Neurol. 2004; Mar. 61(3):239–45. discussion 245-7.
18. Ohno K, Monma S, Suzuki R, Masaoka H, Matsushima Y, Hirakawa K. Saccular aneurysms of the distal anterior cerebral artery. Neurosurgery. 1990; Dec. 27(6):907–12. discussion 912-3.
19. Pandey A, Rosenwasser RH, Veznedaroglu E. Management of distal anterior artery ameurysms: a single institution retrospective analysis (1997-2005). Neurosurgery. 2007; Nov. 61(5):909–16. discussion 916-7.
20. Sekerci Z, Sanli M, Ergün R, Oral N. Aneurysms of the distal anterior cerebral artery: a clinical series. Neurol Neurochir Pol. 2011; Mar-Apr. 45(2):115–20.
21. Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010; Sep. 41(9):1969–77.
22. Sturiale CL, Brinjikji W, Murad MH, Cloft HJ, Kallmes DF, Lanzino G. Endovascular treatment of distal anterior cerebral artery aneurysms: single-center experience and a systematic review. AJNR Am J Neuroradiol. 2013; Dec. 34(12):2317–20.
23. Suh SJ, Kang DG, Ryu KY, Cho JH. Endovascular treatment of ‘kissing aneurysms’ at the anterior communicating artery. J Korean Neurosurg Soc. 2008; Sep. 44(3):163–5.
24. Sun Z, Wu C, Wang F, Xue Z, Xu B, Zhou D. Surgical management of intracranial mirror aneurysms. Zhonghua Wai Ke Za Zhi. 2013; Oct. 51(10):912–5.
25. Suzuki K, Yatomi K, Yamamoto M, Oishi H, Arai H. Endovascular therapy of distal anterior cerebral artery aneurysms: single-institution clinical experience with 47 patients (49 aneurysms). Journal of Neuroendovascular Therapy. 2019; 13:329–35.
26. Yasargil MG, Carter LP. Saccular aneurysms of the distal anterior cerebral artery. J Neurosurg. 1974; Feb. 40(2):218–23.
27. Yasargil MG. Microneurosurgery, in Clinical Considerations, Surgery of the Intracranial Aneurysms and Results (Microsurgical Anatomy of the Basal Cisterns & Vessels of Brain). Vol. 2.New York, NY: Thieme;1984. p. 33–123.
28. Yasargil MG. Surgery of the Intracranial Aneurysms and Results. Microneurosurgery, Vol. II. Clinical Considerations. Stuttgart: George Thieme;1984. p. 224–31.
Fig. 1.
(A) Pre-operative computed tomography (CT) scan showing inter-hemispheric hematoma (black arrow). Pre-operative 3D reconstructed image of digital subtraction angiography brain showing right distal anterior cerebral artery (DACA) aneurysm (B) (white arrow) and left DACA aneurysm (C) (white arrow). Pre-operative CT angiography image showing bilateral DACA aneurysms (D) (black arrows).
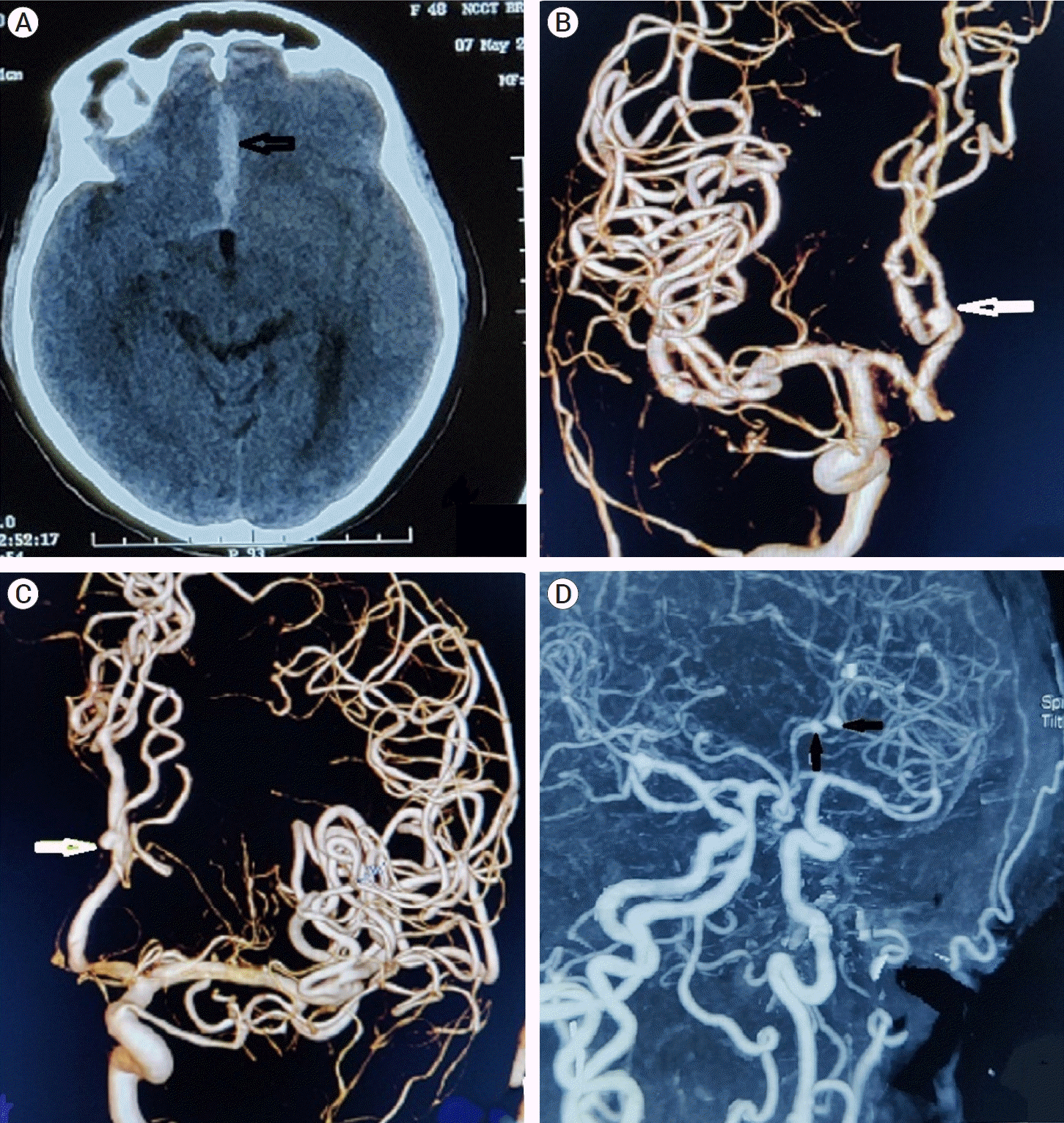
Fig. 2.
Pre-operative 2D images of digital subtraction angiography brain. (A) Antero-posterior view showing right distal anterior cerebral artery (DACA) aneurysm (black arrow), (B) Lateral view showing right DACA aneurysm (black arrow), (C) Antero-posterior view showing left DACA aneurysm (black arrow), (D) Lateral view showing left DACA aneurysm (black arrow).
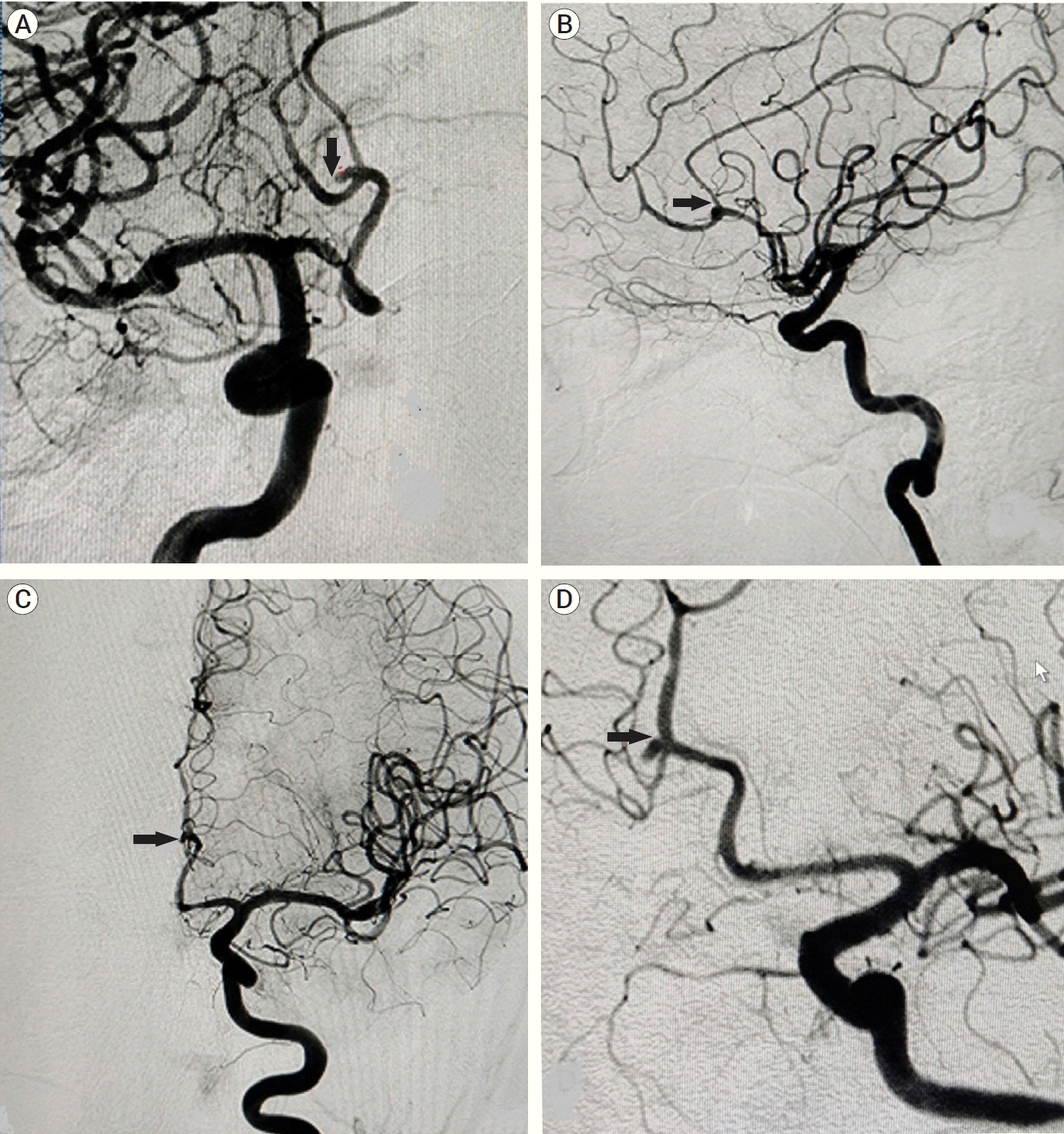
Fig. 3.
(A) Intra-operative microscopic image showing vascular clips applied to bilateral distal anterior cerebral artery DACA aneurysm (black arrows), (B) Intra-operative indocyanine Green video angiography showing the patency of the branches of bilateral distal ACA and complete exclusion of the aneurysm from the circulation, (C) Post-operative CT head after one month showing no residual hematoma. ACA, anterior cerebral artery; CT, computed tomography




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download