Abstract
Background
Percutaneous radiofrequency thermocoagulation (RFTC) has been widely utilized in the management of trigeminal neuralgia. Despite using image guidance, accurate needle positioning into the target area still remains a critical element for achieving a successful outcome. This study was performed to precisely clarify the anatomical information required to ensure that the electrode tip is placed on the sensory component of the mandibular nerve (MN) at the foramen ovale (FO) level.
Results
The cross-sectioned anterior and posterior divisions of the MN at the FO level could be distinguished based on an irregular boundary and color difference. The anterior division was clearly brighter than the posterior one. The anterior division of the MN at the FO level was located at the whole anterior (38.0%), anteromedial (6.0%), anterior center (8.0%), and anterolateral (22.0%) parts. The posterior division was often located at the whole posterior or posterolateral parts of the MN at the FO level. The anterior divisions covered the whole MN except for the medial half of the posterolateral part in the overwrapped images of the cross-sectional areas of the MN at the FO level. The cross-sectional areas of the anterior divisions were similar in males and females, whereas those of the posterior divisions were significantly larger in males (P = 0.004).
Trigeminal neuralgia (TN) is one of the most excruciating disorders in the orofacial region, causing considerable suffering for many afflicted patients. It is characterized by brief recurrent unilateral electric shock-like pain in the distribution area of one or multiple divisions of the trigeminal nerve and is commonly triggered by innocuous stimuli, including wind draughts, light touching, talking, eating, drinking, shaving, washing, and applying makeup [1,2]. It can occur at any age, but previous studies have indicated that most cases occur after the age of 50, some cases can be observed in the second and third decades, but occurrences in children are extremely rare. Women are more commonly affected than men, and the male-to-female prevalence ratio ranges from 1 to 1.5–1.7 [3,4]. TN typically affects one or more divisions of the trigeminal nerve. Many previous studies have found that the maxillary nerve and mandibular nerve (MN) are most commonly involved, with ophthalmic nerve involvement being less common [5–7].
The management options for patients with TN are closely connected with various factors such as age, overall health, severity of the disease, and comorbidities. Treating a pain in TN can pose a substantial clinical challenge, and the initial pharmacological therapy is commonly the preferred primary manner in conservative management. In TN patients who did not respond to medication, it can be effectively managed by using minimally invasive techniques including microvascular decompression, balloon compression, percutaneous radiofrequency thermocoagulation (RFTC), and radiosurgery. Among them, RFTC has been widely utilized in the management of TN, because of its minimal invasiveness and low risk with a high success rate [8]. Vibration and friction from the radiofrequency generator produce heat during RFTC, which can produce thermocoagulation, denaturation, and necrosis of the target nerve through an electrode of the needle.
Among various percutaneous RFTC techniques, the classical Hartel anterior approach has been most often used to treat TN. It allows the tip of the needle to be further advanced into the inner foramen ovale (FO) and eventually reach the trigeminal ganglion [9]. Using this treatment under fluoroscopic guidance has a success rate exceeding 90% [10,11]. However, repetitive repositioning of the needle tip to search for the targeted division in the trigeminal ganglion can damage adjacent divisions or cause other serious complications, such as cerebrospinal fluid leakage, intracranial pneumoniae infections, and hemorrhage [8].
The objective of improving both clinical outcomes and safety has promoted a shift in the treatment strategy in percutaneous RFTC for TN from intracranial localization to selective extracranial localization of individual divisions [12]. The transfer of interventional targets from the intracranial ganglion to the extracranial trigeminal nerve has been reflected in recent studies that used the extracranial within-FO approach to selectively destroy the MN [13–15]. Telischak et al. [13] found that this new approach can provide long-term benefits for the MN similar to those from the Hartel anterior approach, but with less risk of a nonspecific lesion in the other divisions.
The MN contains both motor and sensory nerve fibers, while other trigeminal divisions comprise only sensory fibers [16]. The small motor root passes deep to the ganglion and through the FO to connect with the sensory root from a short main trunk (the MN) just outside the cranial cavity. The MN gives rise to a meningeal branch and the nerve to the medial pterygoid muscle from its medial side, and then divides into the anterior (mainly motor) and posterior (mainly sensory) divisions [16]. The percutaneous within-FO RFTC approach for the MN is also unfortunately an invasive and destructive surgical technique with some reported complications. Masticatory atonia is one of the most common complications, which may be due to the motor nerve fibers in the MN being damaged during the procedure [17]. The electrode tip should only be placed in the sensory component of the MN at the FO level in order to improve outcomes when using the percutaneous within-FO RFTC approach. This study was therefore performed to precisely clarify the anatomical information required to ensure that the electrode tip is placed on the sensory component of the MN at the FO level.
This study used 50 hemi-half heads from 26 South Korean adult cadavers (13 males, 13 females; mean age at death, 78.3 years; age range at death, 50–101 years). None of the cadavers presented any evidence of pathological changes, previous surgical procedures, or traumatic injuries in the target area. This study was approved by the institutional review board of Wonkwang University (IRB ID No. WKIRB-202306-BR-032). All cadavers used in this study were legally donated to the Wonkwang University School of Medicine, and the entire research procedure was strictly conducted according to the ethical standards established in the 1964 Declaration of Helsinki and its later amendments.
The zygomatic arch was exposed after removing the skin, subcutaneous tissue, and adjacent facial muscles such as the zygomaticus major and minor muscles. This arch was cut by using an electric saw for exposing entirety of the temporalis and masseter muscles. The temporalis and masseter muscles were carefully removed, and then the mandibular ramus was sawed vertically along the mandibular notch to expose the inferior alveolar nerve and the nerve to the mylohyoid muscle. The lateral and medial pterygoid muscles were carefully removed without any nerve injuries. For a clearer view, the maxillary vessels were also removed completely. Two divisions of the MN and their branches were carefully and clearly dissected, and a trunk of the MN passing through the FO was exposed (Fig. 1A). This trunk was then cut transversely at the FO level, and photographs were taken with the inclusion of a scalebar to obtain its cross-sectional image (Fig. 1B). The outline of the main trunk and boundaries of the anterior and posterior divisions of the MN were manually drawn using a transparent film on the image (Fig. 1C). These were then measured using digital image analysis software (Image J; National Institutes of Health). Transverse and longitudinal lines were then drawn to divide the cross-section of the MN into four equal parts on the image, and the somatotopic location of each division was analyzed.
Student’s t-test was used to compare the cross-sectional area of the MN between male and female specimens at the FO level. The paired t-test was used to compare that of each division at the FO level. Significance was set at P < 0.05 for Student’s t-test and at P < 0.01 for the paired t-test.
The main trunk of the MN was divided into the anterior and posterior divisions using an irregular boundary. The posterior division tended to be grouped in clusters at the FO level. The sectional morphology of the anterior division presented as a ribbon-like shape at the FO, whereas the posterior division was circular. The color of each division was often inconsistent in the same specimen; it could be easily distinguished by the naked eye that the posterior division was much darker than the anterior division (Fig. 1B).
There were high degrees of variability in the somatotopic location of the anterior division at the FO level across the specimens (Table 1). The anterior division was located within the anterior part of the MN in 37 (74.0%) of the specimens, which included the whole anterior part in 19 specimens (38.0%), the anteromedial part in 3 (6.0%), the anterior center part in 4 (8.0%), and the anterolateral part in 11 (22.0%). In three specimens (6.0%), the anterior division spanned three parts: the whole anterior, partial posterolateral, and partial posteromedial. The anterior division was located at the anteromedial and posteromedial parts of the MN in ten specimens (20.0%). The posterior division was regularly located at the whole posterior or posterolateral part of the MN at the FO level.
The territories of the anterior divisions were identified on the overwrapped cross-sectional areas as follows: The anterior division mostly occupied the whole anterior part of the MN at the FO level in both sexes, whereas the anterior division in the females tended to invade the posteromedial part of the MN than that in the males (Fig. 2A, B). In the overwrapped cross-sectional areas of all specimens, the anterior divisions covered the whole MN except for the medial half of the posterolateral part in both sexes (Fig. 2C).
The cross-sectional area of the MN at the FO level was significantly larger (P = 0.019) in males (19.59 ± 3.52 mm2, mean ± standard deviation) than in females (17.13 ± 3.66 mm2) (Table 2). The cross-sectional area of the posterior division (10.25 ± 2.85 mm2) was also significantly larger than that of the anterior division (8.11 ± 2.02 mm2) (P < 0.001, Table 3). The cross-sectional areas of the anterior divisions were similar in males and females (8.20 ± 1.70 mm2 and 8.02 ± 2.32 mm2, respectively), whereas those of the posterior divisions were significantly larger in males (11.38 ± 3.13 mm2 in males and 9.11 ± 2.01 mm2 in females) (P = 0.004, Table 4). The ratio of the cross-sectional areas of the anterior and posterior divisions was 0.77% in males and 0.90% in females (Table 4).
The trigeminal nerve is the fifth and largest cranial nerve. It originates from the brainstem at the upper border of the ventral surface of the pons as a small motor and large sensory root [16]. The nerve fibers of the sensory root consist of the central processes of sensory neurons whose cell bodies of origin are located within the trigeminal ganglion, while those of the motor root arise from cell bodies located in the pons [18]. After leaving the lateral surface of the body of the pons, the motor and sensory roots course posteromedially under the tentorium cerebelli to reach the trigeminal ganglion, which occupies a concavity in the trigeminal cave, on the floor of the middle cranial fossa. The peripheral processes of the sensory ganglionic neurons form three major branches when they leave the convex ventral surface of the ganglion: the ophthalmic and maxillary nerves, and the sensory part of the MN [19]. The fibers from the motor root course deep to the trigeminal ganglion in the medial cranial fossa and exit the cranial cavity through the FO as the motor portion of the MN [18]. The MN immediately passes between the veli palatini muscles after passing through the FO and provides a meningeal branch and nerve to the medial pterygoid from its medial side. The MN then divides into two divisions: the anterior, comprising mostly motor neurons, and the posterior, comprising mostly sensory ones [16,20–22]. The anterior division gives off three major motor branches, comprised of the masseteric nerve, the deep temporal nerve for the lateral pterygoid, and the sensory buccal nerve. The posterior division is comprised almost entirely of sensory neurons, with its only motor branch being the nerve for the mylohyoid. Its sensory branches include the auriculotemporal, lingual, and inferior alveolar nerves.
The color difference was also clearly observed in the cross-sectioned anterior and posterior divisions of the MN at the FO level in the present study. Myelinated nerve fibers generally have a brighter or whiter appearance than unmyelinated ones, which can be attributed to the presence of the fatty substance within the myelin sheath [23]. The anterior division of the MN therefore appears brighter than the posterior division due to its predominance of myelinated motor nerve fibers. In contrast, the posterior division of the MN primarily comprises sensory nerve fibers that encompass a mixture of myelinated and unmyelinated fibers, resulting in a darker appearance. It has also been observed that the compactness of nerve bundles can influence the color, with a denser and more tightly packed nerve fiber arrangement within a bundle tending to result in a darker appearance. The darker color observed in the posterior division of the MN can therefore be attributed to the denser and more compact nature of its nerve bundles. In contrast, the nerve bundles in the anterior division are sparsely distributed and contain a higher proportion of connective tissue, which may contribute to a lighter appearance. These observations indicate that both the density of nerve fibers within a bundle and the amount of surrounding connective tissue can influence the color difference between the anterior and posterior divisions of the MN.
The anterior and posterior MN divisions are simply described as being small and large, respectively, in some anatomical textbooks [16,20]. In the present study, the cross-sectional area of the posterior division was also significantly larger than that of the anterior division (Table 3), with this difference being greater in males (Table 4).
Percutaneous RFTC of the trigeminal ganglion via the FO has been adopted as an effective treatment for TN, which generates heat in a controlled and minimally invasive manner to selectively destroy sensory nerve fibers [24]. The MN becomes the primary target of this procedure, because it has been reported as one of frequently involved nerves in patients with TN [5–7] and is easy to approach extracranially. For this reason, pain physicians may have a great interest in related anatomical information for an effective outcome. In consideration of the above-mentioned anatomical descriptions, physicians need to carefully consider the motor root passage—especially from the deep part of the trigeminal ganglion to the medial side of the FO—to avoid direct injuries to the motor nerve caused by an uninsulated electrode tip placed intracranially in the trigeminal ganglion.
Various methodologies have been developed for successful percutaneous RFTC, including temperature monitoring, electric stimulation with awake-patient feedback, X-ray fluoroscopy, computed tomography (CT), and neuronavigator control [25,26]. However, some deficiencies remain that cannot be ignored. The most significant may be when the final target is a single division of the trigeminal nerve, especially the MN. Percutaneous RFTC by approaching the trigeminal ganglion via the FO can easily damage adjacent divisions and hence induce corresponding complications [27,28]. The technique of CT-guided selective percutaneous within-FO RFTC for TN only involving the MN was recently found to overcome this significant deficiency [13,14]. Nevertheless, this approach also appears to have its imperfections. Masticatory weakness is one of the common complications encountered in related procedures, and its incidence rate has been reported to be as high as 42% [14]. This may be due to some motor components of the anterior division of the MN going to the masticatory muscles being damaged by the physicians placing the electrode tip itself near to the FO level. This suggests that the incidence of motor function injury may be reduced if the electrode tip can be accurately placed in the posterior division of the MN at the FO level away from the anterior division. However, no obvious comparative data could be found because studies describing the morphology of the MN with respect to similar interventional procedures at the FO level have been extremely rare [29] and only a very small number of studies addressing the angle of needle insertion have been reported [30].
The cross-sectioned anterior and posterior divisions of the MN were differentiated by an irregular boundary at the FO level in the present study (Fig. 1B). No anterior division invaded the medial half of the lateral posterior division when all of the cross-sectional areas of the MNs were overwrapped (Fig. 2C). From a clinical perspective, this finding in the present study can offer good information for deciding an optimal position for the needle at the FO level without damage to the motor components of the MN coursing to the masticatory muscles. When using image guidance such as fluoroscopy or CT, the electrode tip should be placed in the medial half of the posterolateral region in the FO (Fig. 3).
Our study has also some limitations like other cadaver studies. It may be challenging to fully understand specific conditions or complications that can occur in actual patients. Thus, intensive caution is required when interpreting the results of cadaver studies and evaluating their applicability to real patients. Future studies and clinical trials can address this issue based on the findings of the present study.
In conclusion, this study has revealed the area dimensions of the anterior and posterior divisions of the MN at the FO level. The obtained anatomical information is expected to provide a new perspective on the optimal location of an electrode tip at the FO level during percutaneous within-FO RFTC procedures.
ACKNOWLEDGMENTS
The authors thank the body donors and their families; without their unique donations, it would have been impossible for us to complete this study.
Notes
REFERENCES
1. Headache Classification Committee of the International Headache Society. 2018; The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 38:1–211. DOI: 10.1177/0333102417738202.
2. Han KR, Kim C, Kim DW, Cho OG, Cho HW. 2006; Long-term outcome of trigeminal nerve block with alcohol for the treatment of trigeminal neuralgia. Korean J Pain. 19:45–50. DOI: 10.3344/kjp.2006.19.1.45.

3. Lee CH, Jang HY, Won HS, Kim JS, Kim YD. 2021; Epidemiology of trigeminal neuralgia: an electronic population health data study in Korea. Korean J Pain. 34:332–8. DOI: 10.3344/kjp.2021.34.3.332. PMID: 34193639. PMCID: PMC8255158.

4. Kim MS, Ryu YJ, Park SY, Kim HY, An S, Kim SW. 2013; Secondary trigeminal neuralgia caused by pharyngeal squamous cell carcinoma - A case report -. Korean J Pain. 26:177–80. DOI: 10.3344/kjp.2013.26.2.177. PMID: 23614082. PMCID: PMC3629347.

5. Neto HS, Camilli JA, Marques MJ. 2005; Trigeminal neuralgia is caused by maxillary and mandibular nerve entrapment: greater incidence of right-sided facial symptoms is due to the foramen rotundum and foramen ovale being narrower on the right side of the cranium. Med Hypotheses. 65:1179–82. DOI: 10.1016/j.mehy.2005.06.012. PMID: 16084672.

6. Cruccu G, Biasiotta A, Galeotti F, Iannetti GD, Innocenti P, Romaniello A, et al. 2006; Diagnosis of trigeminal neuralgia: a new appraisal based on clinical and neurophysiological findings. Suppl Clin Neurophysiol. 58:171–86. DOI: 10.1016/S1567-424X(09)70067-4. PMID: 16623330.
7. Bangash TH. 2011; Trigeminal neuralgia: frequency of occurrence in different nerve branches. Anesth Pain Med. 1:70–2. DOI: 10.5812/aapm.2164. PMID: 25729659. PMCID: PMC4335746.

8. Kanpolat Y, Savas A, Bekar A, Berk C. 2001; Percutaneous controlled radiofrequency trigeminal rhizotomy for the treatment of idiopathic trigeminal neuralgia: 25-year experience with 1,600 patients. Neurosurgery. 48:524–32. DOI: 10.1097/00006123-200103000-00013. PMID: 11270542.

9. Taub E. Lozano AM, Gildenberg PL, Tasker RR, editors. 2009. Radiofrequency rhizotomy for trigeminal neuralgia. Textbook of stereotactic and functional neurosurgery. Springer;p. 2421–8. DOI: 10.1007/978-3-540-69960-6_141.
10. Gökalp HZ, Kanpolat Y, Tümer B. 1980; Carotid-cavernous fistula following percutaneous trigeminal ganglion approach. Clin Neurol Neurosurg. 82:269–72. DOI: 10.1016/0303-8467(80)90019-0. PMID: 6263532.

11. Tang YZ, Jin D, Li XY, Lai GH, Li N, Ni JX. 2014; Repeated CT-guided percutaneous radiofrequency thermocoagulation for recurrent trigeminal neuralgia. Eur Neurol. 72:54–9. DOI: 10.1159/000357868. PMID: 24853911.

12. Huang Q, Liu X, Chen J, Bao C, Liu D, Fang Z, et al. 2016; The effectiveness and safety of thermocoagulation radiofrequency treatment of the ophthalmic division (V1) and/or maxillary (V2) and mandibular (V3) division in idiopathic trigeminal neuralgia: an observational study. Pain Physician. 19:E1041–7. DOI: 10.36076/ppj/2016.19.E1041.
13. Telischak NA, Heit JJ, Campos LW, Choudhri OA, Do HM, Qian X. 2018; Fluoroscopic C-arm and CT-guided selective radiofrequency ablation for trigeminal and glossopharyngeal facial pain syndromes. Pain Med. 19:130–41. DOI: 10.1093/pm/pnx088. PMID: 28472393.

14. Huang B, Xie K, Chen Y, Wu J, Yao M. 2019; Bipolar radiofrequency ablation of mandibular branch for refractory V3 trigeminal neuralgia. J Pain Res. 12:1465–74. DOI: 10.2147/JPR.S197967. PMID: 31190956. PMCID: PMC6514122.
15. Zeng F, Zhu M, Wan Q, Yan Y, Li C, Zhang Y. 2020; The treatment of V2 + V3 idiopathic trigeminal neuralgia using peripheral nerve radiofrequency thermocoagulation via the foramen rotundum and foramen ovale compared with semilunar ganglion radiofrequency thermocoagulation. Clin Neurol Neurosurg. 196:106025. DOI: 10.1016/j.clineuro.2020.106025. PMID: 32590251.
16. Berry M, Standring SM, Bannister LH. Williams PL, editor. 1995. Nervous system. Gray's anatomy. 38th ed. Churchill Livingstone;p. 901–1397. DOI: 10.37019/e-anatomy/49576.
17. Peris-Celda M, Graziano F, Russo V, Mericle RA, Ulm AJ. 2013; Foramen ovale puncture, lesioning accuracy, and avoiding complications: microsurgical anatomy study with clinical implications. J Neurosurg. 119:1176–93. DOI: 10.3171/2013.1.JNS12743. PMID: 23600929.

18. Fillmore EP, Seifert MF. Tubbs RS, Rizk E, Shoja MM, Loukas M, Barbaro N, Spinner R, editors. 2015. Anatomy of the trigeminal nerve. Nerves and nerve injuries. Vol 1: history, embryology, anatomy, imaging, and diagnostics. Academic Press;p. 319–50. DOI: 10.1016/b978-0-12-410390-0.00023-8.
19. Wilson-Pauwels L, Akesson EJ, Stewart PA. 1998. Cranial nerves: anatomy and clinical comments. B.C. Decker;p. 51–69. DOI: 10.37019/e-anatomy/49563.
20. Romanes GJ. 1981. Cunningham's textbook of anatomy. 12th ed. Oxford University Press;p. 748–56.
21. Woodburne RT, Burkel WE. 1994. Essentials of human anatomy. 9th ed. Oxford University Press;p. 265–8. DOI: 10.5005/jp/books/10278.
22. Snell RS. 2000. Clinical anatomy for medical students. 6th ed. Lippincott Williams & Wilkins;p. 675.
23. Krassioukov AV. Ramachandran VS, editor. 2002. Peripheral nervous system. Encyclopedia of the human brain. 1st ed. Academic Press;p. 817–30. DOI: 10.1016/B0-12-227210-2/00276-4.
24. Singh S, Verma R, Kumar M, Rastogi V, Bogra J. 2014; Experience with conventional radiofrequency thermorhizotomy in patients with failed medical management for trigeminal neuralgia. Korean J Pain. 27:260–5. DOI: 10.3344/kjp.2014.27.3.260. PMID: 25031812. PMCID: PMC4099239.

25. Cheng JS, Lim DA, Chang EF, Barbaro NM. 2014; A review of percutaneous treatments for trigeminal neuralgia. Neurosurgery. 10 Suppl 1:25–33. Erratum in: Neurosurgery 2014; 10 Suppl 2: 372. DOI: 10.1227/NEU.00000000000001687. PMID: 24509496.

26. Ding W, Chen S, Wang R, Cai J, Cheng Y, Yu L, et al. 2016; Percutaneous radiofrequency thermocoagulation for trigeminal neuralgia using neuronavigation-guided puncture from a mandibular angle. Medicine (Baltimore). 95:e4940. DOI: 10.1097/MD.0000000000004940. PMID: 27749549. PMCID: PMC5059051.

27. Toda K. 2008; Operative treatment of trigeminal neuralgia: review of current techniques. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 106:788–805. 805.e1-6DOI: 10.1016/j.tripleo.2008.05.033. PMID: 18657454.

28. Fraioli MF, Cristino B, Moschettoni L, Cacciotti G, Fraioli C. 2009; Validity of percutaneous controlled radiofrequency thermocoagulation in the treatment of isolated third division trigeminal neuralgia. Surg Neurol. 71:180–3. DOI: 10.1016/j.surneu.2007.09.024. PMID: 18291496.

29. Cho KH, Shah HA, Schimmoeller T, Machado AG, Papay FA. 2020; An anatomical study of the foramen ovale for neuromodulation of trigeminal neuropathic pain. Neuromodulation. 23:763–9. DOI: 10.1111/ner.13140. PMID: 32243026.

30. Zdilla MJ, Hatfield SA, Mangus KR. 2016; Angular relationship between the foramen ovale and the trigeminal impression: percutaneous cannulation trajectories for trigeminal neuralgia. J Craniofac Surg. 27:2177–80. DOI: 10.1097/SCS.0000000000003138. PMID: 28005784. PMCID: PMC5266502.
Fig. 1
Photographs showing the methodology for investigating the cross-sectional morphology of the mandibular nerve (MN) at the foramen ovale (FO) level. (A) The MN and all of its branches are exposed in the infratemporal fossa after removing the skin, subcutaneous tissue, zygomatic arch, ramus of mandible, masticatory muscles, and maxillary vessels, etc. A red-colored dotted circle indicates the location of the FO. (B) After cutting transversely the short trunk close to the FO, the cross-sectional area of each division could be distinguished by an irregular boundary and color in most of the specimens. The posterior division (asterisk) is clearly darker than the anterior one. (C) The boundary of each division of the MN is manually drawn using a transparent film on the image. AD: anterior division, Ant: anterior, ATN: auriculotemporal nerve, BN: buccal nerve, DTN: deep temporal nerve, IAN: inferior alveolar nerve, Lat: lateral, LN: lingual nerve, LPN: nerve to lateral pterygoid muscle, LPP: lateral plate of the pterygoid process, Med: medial, MLN: nerve to mylohyoid muscle, MsN: masseteric nerve, PD: posterior division, Post: posterior.
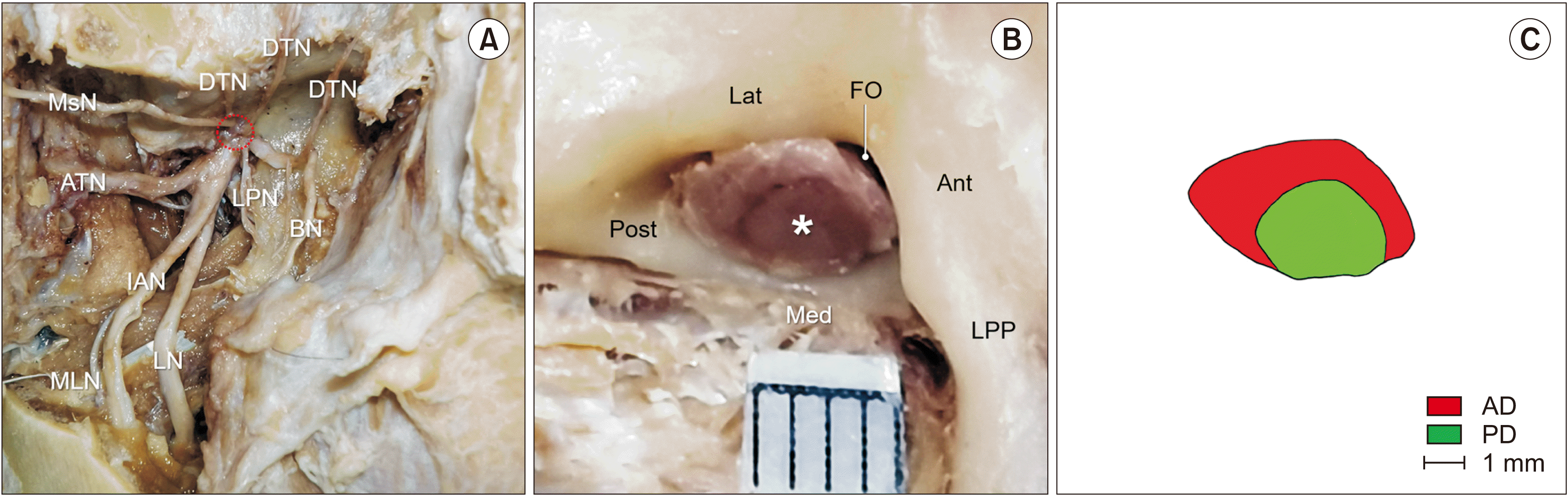
Fig. 2
Schematic drawing of the territory of the anterior division in the cross-sectional area of the mandibular nerve (MN) at the foramen ovale (FO) level in males (A), females (B), and all specimens (C). Dashed lines indicate imaginary lines quadrisecting the cross-sectional area of the MN. Darker color indicates more-overwrapped areas of the anterior division. Unfilled areas indicate the posterior division of the MN where the anterior division is not invaded. Ant: anterior, Lat: lateral, Med: medial, Post: posterior.
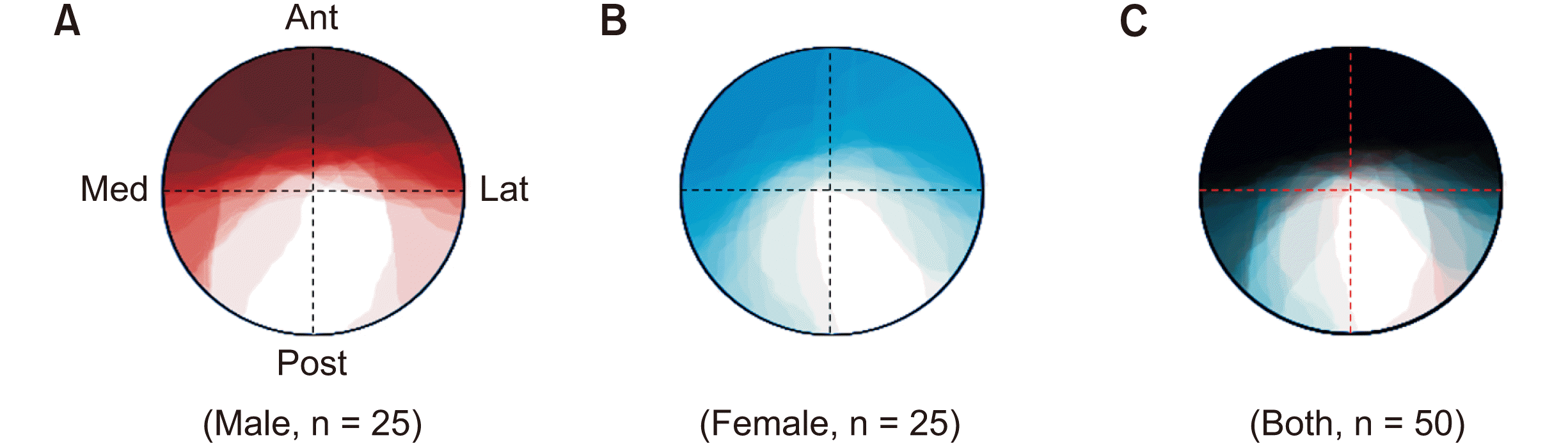
Fig. 3
A fluoroscopic image showing an optimal needle positioning in the patient with TN during percutaneous RFTC, and enlarged view of which is shown in the rectangle. A dotted circle, vertical and horizontal lines indicate the FO itself and imaginary lines quadrisecting the area of the FO, respectively. TN: trigeminal neuralgia, RFTC: radiofrequency thermocoagulation, FO: foramen ovale.
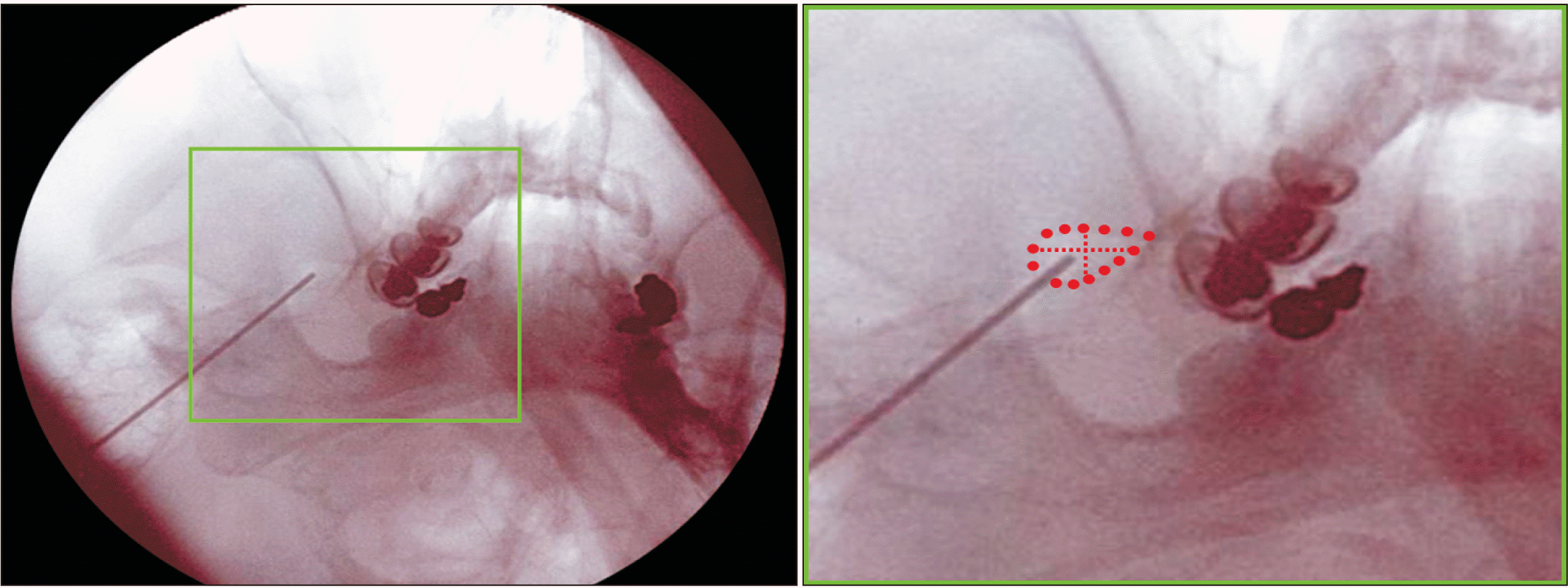
Table 1
Somatotopic location of the anterior division of the MN at the FO level
Table 2
Cross-sectional area of the MN at the FO level by sex
(mm2)
| Sex (n) | Mean | SD | Max | Min |
|---|---|---|---|---|
| Male (25) | 19.59* | 3.52 | 26.70 | 12.41 |
| Female (25) | 17.13 | 3.66 | 24.59 | 11.30 |
Table 3
Cross-sectional areas of the anterior and posterior divisions at the FO level
(mm2)
| Division | Mean | SD | Max | Min |
|---|---|---|---|---|
| Anterior | 8.11 | 2.02 | 11.98 | 4.29 |
| Posterior | 10.25** | 2.85 | 20.09 | 5.29 |
Table 4
Ratio of the cross-sectional areas of the anterior and posterior divisions (A/P) at the FO level by sex
(mm2)
| Sex (n) | Division | Mean ± SD | A/P (%) |
|---|---|---|---|
| Male (25) | Anterior | 8.20 ± 1.70 | 0.77 |
| Posterior | 11.38 ± 3.13* | ||
| Female (25) | Anterior | 8.02 ± 2.32 | 0.90 |
| Posterior | 9.11 ± 2.01 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download