This article has been retracted. See "Retraction: Comparison of the efficacy of genicular nerve phenol neurolysis and radiofrequency ablation for pain management in patients with knee osteoarthritis" in Volume 38 on page 86.
Abstract
Background
Genicular nerve neurolysis with phenol and radiofrequency ablation (RFA) are two interventional techniques for treating chronic refractory knee osteoarthritis (KOA) pain. This study aimed to compare the efficacy and adverse effects of both techniques.
Methods
Sixty-four patients responding to diagnostic blockade of the superior medial, superior lateral, and inferior medial genicular nerve under ultrasound guidance were randomly divided into two groups Group P (2 mL phenol for each genicular nerve) and Group R (RFA 80°C for 60 seconds for each genicular nerve). The numeric rating scale (NRS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) were used to evaluate the effectiveness of the interventions.
Results
RFA and phenol neurolysis of the genicular nerves provided effective analgesia within groups at 1 week, 1 month, and 3 months compared to baseline. There was no significant difference between the groups in terms of NRS and WOMAC scores at all measurement times. At the 3rd month follow-up, 50% or more pain relief was observed in 53.1% of patients in Group P and 50% of patients in Group R. The rate of transient paresthesia was 34.4% in Group P and 6.3% in Group R, and this was significantly higher in Group P.
Chronic knee pain is a common condition that affects many people, particularly those with knee osteoarthritis (KOA). It can limit mobility, reduce the quality of life, and increase the risk of falling. The worldwide prevalence of symptomatic KOA in individuals over 60 years of age is 9.6% in men and 18% in women. Most (80%) patients with KOA have limited mobility, and 25% cannot perform major activities of daily living [1]. In 2019, osteoarthritis (OA) was the 15th most common cause of disability and labor loss [2].
There are various treatment options for KOA pain, including medications, physical therapy, injections, and surgery. However, some patients may not respond to these treatments or may experience adverse effects. Moreover, the cost of total knee arthroplasty can be very high, and it may not be accessible in all countries. One alternative treatment option is genicular nerve ablation, which involves destroying nerves that carry pain signals from the knee joint to the brain. Genicular nerve ablation can be performed using radiofrequency ablation (RFA) or chemical agents such as phenol [3,4]. Both methods aim to provide long-term pain relief and functional improvement in patients with chronic knee pain.
Genicular nerve RFA is a procedure that uses heat to create lesions on the genicular nerves. It is an effective and safe treatment for chronic knee pain due to KOA [5]. However, it requires expensive specialized equipment and trained personnel, which may not be available or affordable in some settings. Phenol is used to chemically destroy the genicular nerves. This is a low-cost and straightforward technique that can be performed using readily available materials and skills. Both procedures can be performed under ultrasound (US) guidance to identify target nerves and avoid damage to nearby structures [6].
The primary aim of this study was to compare the efficacy of genicular phenol and radiofrequency applications in managing KOA pain. The secondary aim was to determine the side effects and complications of these two methods.
Approval for this prospective study was obtained from the local ethics committee of Ankara Etlik City Hospital (number 2023-209). This study was registered at ClinicalTrials.gov (registration number NCT05908942). Written informed consent was obtained from patients for interventional procedures and permission to participate in the study. A computer-assisted randomization program was used to categorize the patients into groups.
The inclusion criteria were moderate to severe knee pain due to KOA (pain of intensity six or more on a numeric rating scale [NRS] of 0–10), persistent pain for more than six months, grade 3 or 4 OA in the radiological Kellgren–Lawrence (KL) classification, and failure to manage pain with conservative methods such as analgesics, intra-articular injection, and physical therapy. The exclusion criteria were knee pain due to causes other than KOA (such as meniscopathy, trauma, spine disorder, or rheumatoid arthritis), previous knee surgery, intra-articular injection in the last 3 months, coagulopathy, use of antiaggregants or anticoagulants other than acetylsalicylic acid 100 mg, infection, severe psychiatric illness, having a cardiac pacemaker, and renal-hepatic insufficiency.
All procedures were performed under sterile conditions. Patients were placed in the supine position and monitored according to the American Society of Anesthesiologists standards. Vascular access was established. The superior medial genicular nerve (SMGN), superior lateral genicular nerve (SLGN), and inferior medial genicular nerve (IMGN) were targeted for the blockade.
For SMGN and SLGN, a 5–12 MHz linear US probe (LOGIQ P9; GE Ultrasound) was placed parallel to the long axis of the femur at the junction of the shaft and epicondyle. The US probe was moved caudally to visualize the genicular artery at the junction of the shaft and epicondyle just above the periosteum under the vastus medialis and vastus lateralis muscles. The junction of the tibial shaft and medial epicondyle was targeted for the IMGN. The US probe was moved cranially to visualize the genicular artery below the medial collateral ligament. Doppler imaging confirmed blood flow in the genicular artery. Skin and subcutaneous tissues were anesthetized with 1 mL of 2% lidocaine using a 27 gauge needle. In the same plane as the US probe, a 22 gauge spinal needle was inserted to reach the genicular nerve adjacent to the genicular artery, and 2 mL of a mixture of 0.25% bupivacaine and 1% lidocaine was injected for each genicular nerve after negative aspiration. Patients with 50% or more pain reduction for at least 3 hours after diagnostic blockade were subjected to a neurolytic block or RFA. For phenol neurolysis and RFA, the technique described for diagnostic blockade was used. Probe placement for the SMGN, SLGN, and IMGN is shown in Fig. 1.
For phenol neurolysis, 2 mL of 6% phenol solution prepared from crystallized phenol was applied to each genicular nerve. Because of the local anesthetic effect of phenol and the painlessness of the injection, no local anesthetic was administered beforehand.
For RFA, a 20-gauge, 10 mm active type, and 100 mm RF cannulas (TOP Nuropole Needle; TOP Corporation) and an RF generator (TOP Lesion Generator; TOP Corporation) were used. For sensory stimulation, 0.5 Volt (V) current was applied at a frequency of 50 Hz, and a paresthesia response was obtained in the relevant knee joint region. The needle position was confirmed by increasing the current to twice the voltage at which the sensory stimulation was obtained, and no motor response was observed at a frequency of 2 Hz. Each genicular nerve was injected with 2 mL of 2% lidocaine before RFA at 80°C for 60 seconds.
The patients were observed for 2 hours after the interventional procedures for possible complications and adverse events. Patients were offered paracetamol oral tablets for post-procedure pain, with a maximum daily dose of 3 g.
The patients were evaluated before the procedure, and at one week, one month, and three months after the procedure. The assessment was performed by a pain specialist who had not seen the patients before and had no knowledge of which procedure was performed. The NRS and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) were used for assessment. For the NRS, the assessment inquired about the most painful period, such as climbing stairs (0: no pain and 10: highest pain). The WOMAC score is a self-administered questionnaire that measures the symptoms and physical disability of patients with hip or KOA. It consists of 24 items that cover three dimensions: pain, stiffness, and physical function. The items were scored on a scale of 0 to 4, with higher scores indicating worse outcomes. The score range for pain is 0–20, stiffness is 0–8, and physical function is 0–68. The WOMAC score is a reliable, valid, and responsive tool for assessing OA patients [7]. The primary outcome was the response to treatment, measured using the WOMAC. The secondary outcomes were NRS scores, the proportion of unexpected events, and the side effects in each group. Unexpected events and side effects (paresthesia, motor deficits, and increase in pain) related to the interventions were recorded at each visit. Meaningful pain relief is defined as a > 50% reduction in pain.
The sample size was calculated using the G*Power 3.1.9.4 software program with an effect size of 0.831, α = 0.05, and power (1-β) = 0.95. A total of 32 participants were included in each group. For this analysis, the statistically significant WOMAC data at month 1 in the study (29.16 ± 8.66 for the steroid group, 37.53 ± 11.45 for the RFA group) by Sarı et al. [8] was used.
All analyses were performed using SPSS version 25.0 (IBM Corp.). Kolmogorov–Smirnov and Shapiro–Wilk tests were used to determine the presence of a parametric distribution. Variables with parametric distribution are shown as the mean (standard deviation) and median (min–max range), and variables with nonparametric distribution are shown as the median (min–max). Numeric variables with normal distribution were compared using an independent samples t-test, and non-normally distributed variables were analyzed with the Mann–Whitney U-test between the groups. Two related intragroup measurements of variables with or without parametric distribution were compared using the paired sample t-test or Wilcoxon signed-rank test, respectively. Categorical variables were compared using the chi-square Fisher’s exact test. Friedman and repeated measures analysis of variance (ANOVA) tests were performed for intragroup comparisons of repeated measurements. Two-way ANOVA was used to determine the group effect on repeated measurements of the parametric distribution. Binary logistic regression was used to determine possible associations between variables and meaningful pain relief. Statistical significance was set at P < 0.05.
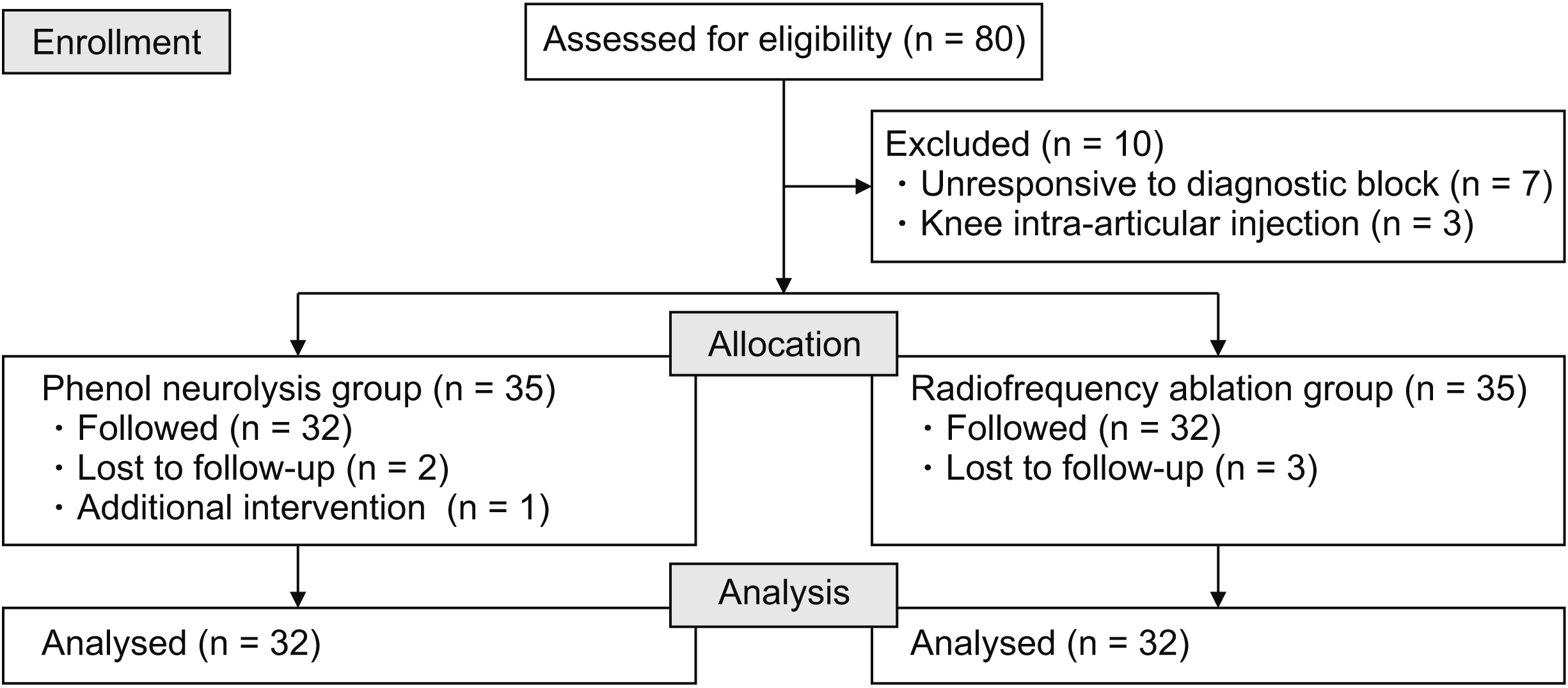
Eighty patients who met the inclusion criteria were included in the study for diagnostic blockade. Seven patients did not respond to the diagnostic blockade, and three patients were excluded from the study because of intra-articular knee injection by an orthopedic clinic. After excluding patients lost to follow-up and those who underwent additional intervention for knee pain, the study was completed with 32 patients in each group: the phenol neurolysis group (Group P) and the RFA group (Group R). A patient flowchart is shown in Fig. 2.
Demographic and clinical features of the patients are presented in Table 1. The groups were similar in age, gender, body mass index, KL grade, duration of pain, diabetes mellitus and side of treatment.
Paresthesia after treatment was significantly higher in Group P (P = 0.011). Motor deficit was not observed in any of the patients in either group. An increase in pain after treatment was seen in 6.3% (2/32) of the patients in Group P, but no patient reported an increase in pain in Group R (Table 2).
The NRS and WOMAC scores for both groups are shown in Table 3. The NRS and WOMAC scores evaluated at different visits were similar in both the groups. Intragroup comparisons of NRS scores revealed significant differences between baseline to 1st week, baseline to 1st month, and baseline to 3rd month in both groups (For all comparisons, P < 0.001). Intragroup comparisons of WOMAC scores revealed significant differences between baseline to 1st week, baseline to 1st month, and baseline to 3rd month in both groups (P < 0.001). The NRS and WOMAC scores over time for both groups are shown in Fig. 3.
No statistically significant difference was found in meaningful pain relief between the groups for all evaluations.
Both methods of genicular nerve neurolysis effectively reduce chronic knee pain and improve function and quality of life in patients with KOA who are not candidates for or have failed conservative treatments. Both phenol neurolysis and RFA of the genicular nerves significantly improved NRS and WOMAC scores at all measurement times compared with pre-treatment. In the present study, the success rate of achieving a 50% or greater reduction in pain score in Group P was 62.5% at one month, while this rate was 56.3% in Group R, and there was no significant difference. At three months, this rate was 53.1% in Group P and 50% in Group R. Risso et al. [3] achieved a 46% success rate at six months with genicular phenol neurolysis in KOA. In a double-blind, randomized controlled study by Choi et al. [5], the 50% success rate with genicular RFA was 59% at three months. In a systematic review evaluating the efficacy of genicular RFA, at least 50% success rates at six months were reported to be between 49% and 74% [9].
Despite similarities in the success rates, there are some differences between phenol neurolysis and RFA in terms of complications, duration, and cost. The advantages and disadvantages of genicular phenol neurolysis versus genicular RFA need to be better established, as there is limited evidence comparing these two modalities. Genicular phenol neurolysis is a relatively simple and low-cost procedure that can be performed under US guidance. While the cost per patient of obtaining liquid phenol for genicular phenol neurolysis is approximately 1 euro in the authors’ country, the cost of one spinal needle is 2 euros. In contrast, the cost of one radiofrequency cannula is approximately 120 euros. Genicular RFA is a more complex and expensive procedure that requires specialized equipment and training.
Phenol neurolysis and RFA are generally safe and well tolerated, with low complications and adverse effects rates. However, phenol neurolysis may have a higher risk of causing nerve injury or neuritis than RFA because it can spread beyond the target area and affect adjacent structures. Lesion size in genicular RFA depends on several factors and averages 0.5–1 cm³ [10]. It has been shown that RFA lesion sizes can be reached with a 1 mL dye injection [11]. While this allows liquid agents such as phenol to affect a larger neuronal structure and increase their efficacy, it may also increase the incidence of side effects. In one study, 4 mL of phenol administered to the genicular nerve was detected posterior to the knee [12]. Risso et al. [3] adminestered 1.5 mL of phenol per genicular nerve for genicular nerve neurolysis and achieved a 46% success rate and reported no side effects. In the literature, the suggested range for the use of neurolytic agent was in the range of 0.5–2 mL per genicular nerve [13]. Therefore, 2 mL phenol per geniculate nerve was used to increase efficacy and reported adverse events. In this study, the rate of paresthesia was 34.4% in Group P and 6.3% in Group R. Despite this high rate in Group P, this effect lasted 15 days in the patient who lasted the longest and did not require treatment. In Group P, two patients reported a transient increase in pain after the procedure, the longest lasting two days. No motor block was observed in any of the patients. RFA may have a higher risk of causing skin burns or infection than phenol, as it involves puncturing the skin and inserting electrodes [14]. The present study did not observe such an effect in Group R.
Another difference between phenol and RFA is the duration of the procedure and the pain felt by the patient during the procedure. In this study, RFA was performed for 1 minute after needle insertion, and although local anesthetic was administered before ablation, the procedure was painful. Phenol injection was much shorter after needle insertion, and the procedure was generally painless because of the local anesthetic effect of phenol [15]. A quicker and painless phenol injection is associated with a lower risk of needle displacement from the target tissue than RFA. Thus, distancing the needle tip from the genicular nerve could be prevented. Despite the advantage of phenol among chemical neurolytics with less injection pain, there is no consensus in the literature regarding the ideal chemical neurolytic agent and dose. The effect of phenol starts slowly, and its full effect may sometimes take up to 1 week [13]. Alcohol is another agent used for genicular neurolysis [16]. Although alcohol is a more potent neurolytic agent, the most serious difficulty with its use is injection pain, which can be very severe [17].
Recent studies have shown that many nerves innervate the knee joint. Approximately seven nerves have been identified in the innervation [18]. The superolateral quadrant was innervated by the nerve to the vastus lateralis, the nerve to the vastus intermedius, the superior lateral genicular and common fibular nerves; the inferolateral by the inferior lateral genicular and recurrent fibular nerves (RFN); the superomedial by the nerve to vastus medialis, nerve to vastus intermedius and SMGN; and the inferomedial by the IMGN. The inferomedial quadrant also received innervation from the infrapatellar branch of saphenous nerve (IPBSN). To the best of the authors’ knowledge, no study has applied the ablative method to all branches. The most commonly ablated nerves are the SMGN, SLGN, and IMGN. Kose et al. [19] investigated predictive factors for successful genicular RFA treatment. They targeted the RFN and the IPBSN in addition to RFA of these three primary nerves. However, they applied pulsed radiofrequency at 42°C to the RFN and IPBSN instead of RFA. Due to its proximity to motor branches (such as the inferolateral genicular nerve) and insufficient studies in the literature, the authors only applied neurolysis to the three best-defined main branches.
Although there are anatomical variations of the genicular nerve’s location, recent anatomical evidence supports the effectiveness of classical landmarking for genicular nerve RFA [18,20]. US-guided genicular nerve neurolysis can provide a similar analgesic effect to fluoroscopic-guided genicular nerve neurolysis and reduce the risk of arterial injury [21]. In addition, US does not cause radiation exposure, which can be an advantage over fluoroscopy [22]. Based on these considerations and the authors’ results, US-guided genicular nerve phenol neurolysis may be an alternative to fluoroscopy-guided genicular nerve RFA.
Our study has several limitations. The WOMAC score subgroups, which include pain, stiffness, and physical function, were not included separately in the study, and analysis within the subgroups could not be performed. Therefore, the specific effects of the interventions on stiffness and physical function could not be evaluated. Another limitation is that the authors could not assess the effects of neurolysis methods on analgesic consumption.
In conclusion, although paresthesia was more common with genicular phenol administration, this adverse effect did not require treatment. Genicular nerve neurolysis with phenol and RFA are both effective options for treating chronic KOA pain. RFA is expensive and requires expertise. Phenol neurolysis may be suitable for patients who cannot afford or access RFA, or have contraindications for RFA (such as cardiac pacemakers). More research is needed to compare the long-term outcomes, cost-effectiveness, and safety of these procedures.
Notes
DATA AVAILABILITY
Data files are available from Harvard Dataverse: https://doi.org/10.7910/DVN/TLNAUR.
REFERENCES
1. Neogi T. 2013; The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 21:1145–53. DOI: 10.1016/j.joca.2013.03.018. PMID: 23973124. PMCID: PMC3753584.

2. Haider MZ, Bhuiyan R, Ahmed S, Zahid-Al-Quadir A, Choudhury MR, Haq SA, et al. 2022; Risk factors of knee osteoarthritis in Bangladeshi adults: a national survey. BMC Musculoskelet Disord. 23:333. DOI: 10.1186/s12891-022-05253-5. PMID: 35395747. PMCID: PMC8991964. PMID: a54da1b08f0548bbb9f781b0aec68291.

3. Risso RC, Ferraro LHC, Nouer Frederico T, Peng PWH, Luzo MV, Debieux P, et al. 2021; Chemical ablation of genicular nerve with phenol for pain relief in patients with knee osteoarthritis: a prospective study. Pain Pract. 21:438–44. DOI: 10.1111/papr.12972. PMID: 33277760.

4. Conger A, Gililland J, Anderson L, Pelt CE, Peters C, McCormick ZL. 2021; Genicular nerve radiofrequency ablation for the treatment of painful knee osteoarthritis: current evidence and future directions. Pain Med. 22(Suppl 1):S20–3. DOI: 10.1093/pm/pnab129. PMID: 34308957.

5. Choi WJ, Hwang SJ, Song JG, Leem JG, Kang YU, Park PH, et al. 2011; Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain. 152:481–7. DOI: 10.1016/j.pain.2010.09.029. PMID: 21055873.

6. Guven Kose S, Kirac Unal Z, Kose HC, Celikel F, Akkaya OT. 2023; Ultrasound-guided genicular nerve radiofrequency treatment: prospective randomized comparative trial of a 3-nerve protocol versus a 5-nerve protocol. Pain Med. 24:758–67. DOI: 10.1093/pm/pnad025. PMID: 36869680.

7. Salehi R, Valizadeh L, Negahban H, Karimi M, Goharpey S, Shahali S. 2023; The Western Ontario and McMaster Universities Osteoarthritis, Lequesne Algofunctional index, Arthritis Impact Measurement Scale-short form, and Visual Analogue Scale in patients with knee osteoarthritis: responsiveness and minimal clinically important differences. Disabil Rehabil. 45:2185–91. DOI: 10.1080/09638288.2022.2084776. PMID: 35695001.

8. Sarı S, Aydın ON, Turan Y, Özlülerden P, Efe U, Kurt Ömürlü İ. 2018; Which one is more effective for the clinical treatment of chronic pain in knee osteoarthritis: radiofrequency neurotomy of the genicular nerves or intra-articular injection? Int J Rheum Dis. 21:1772–8. DOI: 10.1111/1756-185X.12925. PMID: 27515095.

9. Fogarty AE, Burnham T, Kuo K, Tate Q, Sperry BP, Cheney C, et al. 2022; The effectiveness of fluoroscopically guided genicular nerve radiofrequency ablation for the treatment of chronic knee pain due to osteoarthritis: a systematic review. Am J Phys Med Rehabil. 101:482–92. DOI: 10.1097/PHM.0000000000001813. PMID: 35006653.

10. Cosman ER Jr, Dolensky JR, Hoffman RA. 2014; Factors that affect radiofrequency heat lesion size. Pain Med. 15:2020–36. DOI: 10.1111/pme.12566. PMID: 25312825.

11. Cushman DM, Monson N, Conger A, Kendall RW, Henrie AM, McCormick ZL. 2019; Use of 0.5 mL and 1.0 mL of local anesthetic for genicular nerve blocks. Pain Med. 20:1049–52. DOI: 10.1093/pm/pny277. PMID: 30590798.

12. González Sotelo V, Maculé F, Minguell J, Bergé R, Franco C, Sala-Blanch X. 2017; Ultrasound-guided genicular nerve block for pain control after total knee replacement: preliminary case series and technical note. Rev Esp Anestesiol Reanim. 64:568–76. DOI: 10.1016/j.redar.2017.04.001. PMID: 28554709.

13. Walega DR, McCormick ZL. 2018; Chemical neurolysis of the genicular nerves for chronic knee pain: reviving an old dog and an old trick. Pain Med. 19:1882–4. DOI: 10.1093/pm/pny023. PMID: 29514315.

14. Kidd VD, Strum SR, Strum DS, Shah J. 2019; Genicular nerve radiofrequency ablation for painful knee arthritis: the why and the how. JBJS Essent Surg Tech. 9:e10. DOI: 10.2106/JBJS.ST.18.00016. PMID: 31333900. PMCID: PMC6635137.

15. D'Souza RS, Warner NS. 2023. Phenol nerve block. StatPearls Publishing;DOI: 10.1007/978-3-642-28753-4_101374.
16. Dass RM, Kim E, Kim HK, Lee JY, Lee HJ, Rhee SJ. 2019; Alcohol neurolysis of genicular nerve for chronic knee pain. Korean J Pain. 32:223–7. DOI: 10.3344/kjp.2019.32.3.223. PMID: 31257831. PMCID: PMC6615444.

17. Tan YL, Neo EJR, Wee TC. 2022; Ultrasound-guided genicular nerve blockade with pharmacological agents for chronic knee osteoarthritis: a systematic review. Pain Physician. 25:E489–502. PMID: 35793174.
18. Tran J, Peng PWH, Lam K, Baig E, Agur AMR, Gofeld M. 2018; Anatomical study of the innervation of anterior knee joint capsule: implication for image-guided intervention. Reg Anesth Pain Med. 43:407–14. DOI: 10.1097/AAP.0000000000000778. PMID: 29557887.
19. Kose SG, Kose HC, Celikel F, Akkaya OT. 2022; Predictive factors associated with successful response to utrasound guided genicular radiofrequency ablation. Korean J Pain. 35:447–57. DOI: 10.3344/kjp.2022.35.4.447. PMID: 36175344. PMCID: PMC9530687.

20. Tran J, Peng P, Agur A. 2020; Evaluation of nerve capture using classical landmarks for genicular nerve radiofrequency ablation: 3D cadaveric study. Reg Anesth Pain Med. 45:898–906. DOI: 10.1136/rapm-2020-101894. PMID: 32928998.

21. Kim DH, Lee MS, Lee S, Yoon SH, Shin JW, Choi SS. 2019; A prospective randomized comparison of the efficacy of ultrasound- vs fluoroscopy-guided genicular nerve block for chronic knee osteoarthritis. Pain Physician. 22:139–46. DOI: 10.36076/ppj/2019.22.139. PMID: 30921977.
22. Sarı S, Aydın ON, Turan Y, Şen S, Özlülerden P, Ömürlü İK, et al. 2017; Which imaging method should be used for genicular nerve radio frequency thermocoagulation in chronic knee osteoarthritis? J Clin Monit Comput. 31:797–803. DOI: 10.1007/s10877-016-9886-9. PMID: 27142099.

Fig. 1
Probe placement for superior medial genicular nerve, superior lateral genicular nerve, and inferior medial genicular nerve. SMGA: superior medial genicular artery, SLGA: superior lateral genicular artery, IMGA: inferior medial genicular artery.
Fig. 3
(A) Numeric rating scale (NRS) and (B) Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores of the groups before and after the treatment. The NRS is shown as the median (min-max range), and WOMAC score is shown as the mean ± standard deviation. aP < 0.001 for NRS-1st week, 1st month and 3rd month compared to NRS-baseline. bP < 0.001 for WOMAC-1st week, WOMAC-1st month and WOMAC-3rd month compared to WOMAC-baseline.
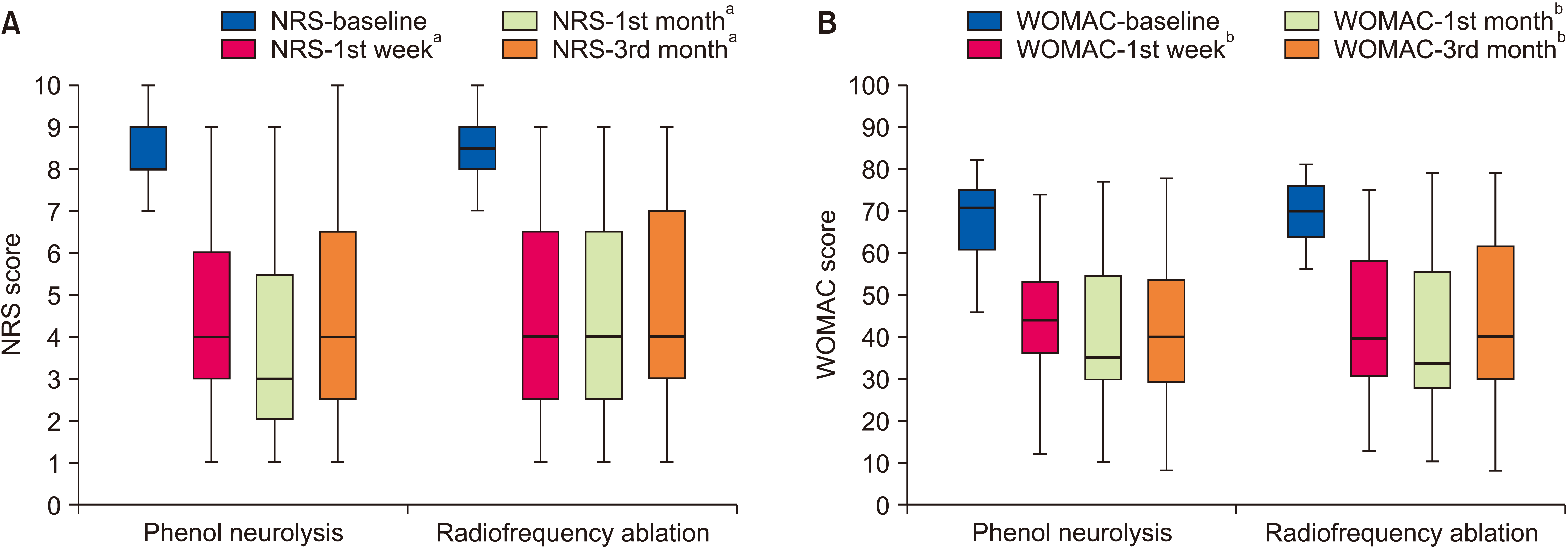
Table 1
The demographic and clinical features of the patients
Table 2
Procedure-related adverse events
| Adverse events |
Group P (n = 32) |
Group R (n = 32) |
P value | |
|---|---|---|---|---|
| Paresthesia | 11 (34.4) | 2 (6.3) | 0.011 | |
| Motor deficit | - | - | ||
| Increase in pain | 2 (6.3) | - |
Table 3
Numeric rating scale (NRS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores of the groups
| NRS and WOMAC scores |
Group P (n = 32) |
Group R (n = 32) |
P value | |
|---|---|---|---|---|
| NRS | ||||
| Baseline | 8.0 (7.0–10.0) | 8.5 (7.0–10.0) | 0.325 | |
| 1st week | 4.0a (1.0–9.0) | 4.0a (1.0–9.0) | 0.859 | |
| 1st month | 3.0a (1.0–9.0) | 4.0a (1.0–9.0) | 0.421 | |
| 3rd month | 4.0a (1.0–10.0) | 4.5a (1.0–9.0) | 0.729 | |
| WOMAC | ||||
| Baseline | 68.0 ± 9.1 | 69.8 ± 7.5 | 0.382 | |
| 1st week | 43.8 ± 15.7a | 42.1 ± 19.1a | 0.706 | |
| 1st month | 40.1 ± 18.9a | 40.9 ± 19.9a | 0.870 | |
| 3rd month | 42.1 ± 18.4a | 43.7 ± 18.9a | 0.725 |