TO THE EDITOR: Posterior reversible encephalopathy syndrome (PRES) is characterized by a myriad of neurological manifestations including renal failure, blood pressure fluctuations, cytotoxic drugs, autoimmune disorders, and preeclampsia or eclampsia. Vasogenic edema associated with PRES results from the disruption of cerebral blood flow autoregulation. Common causes of drug-induced PRES include medications that target receptors controlling vascular permeability or modulate the immunological response [1]. Dasatinib is a potent oral tyrosine kinase inhibitor that inhibits BCR-ABL as well as SRC family kinases [2]. A 29-year-old man receiving dasatinib for chronic myeloid leukemia (CML) treatment presented with generalized tonic-clonic seizure, along with evidence of raised intracranial tension. Magnetic resonance imaging (MRI) of the brain revealed T2/FLAIR hyperintense signals in the parietal and occipital regions. A diagnosis of dasatinib-related atypical PRES was established, and the medication was subsequently discontinued. To the best of our knowledge, this is the first report of atypical PRES associated with dasatinib use.
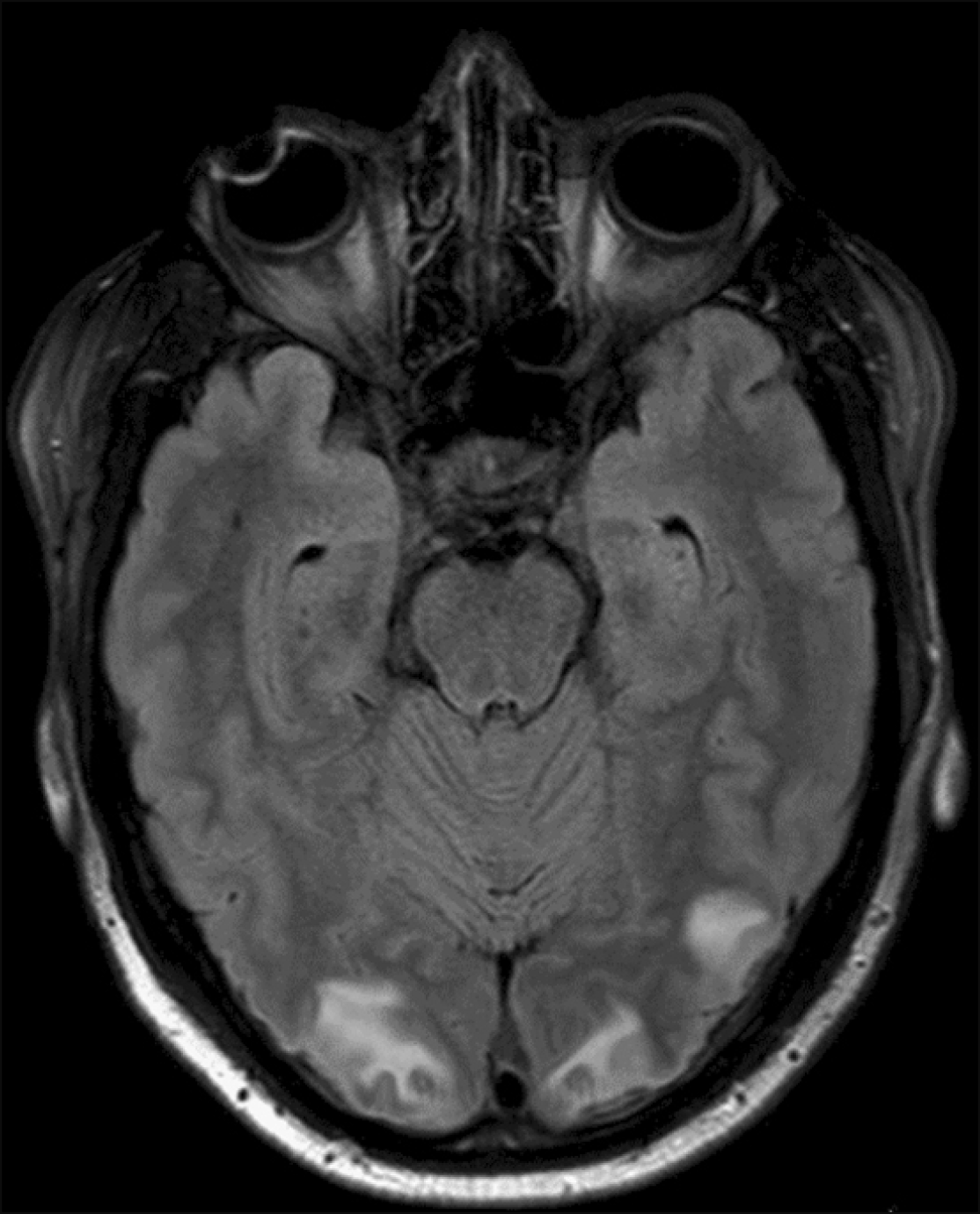
A 29-year-old man was diagnosed with CML in February 2016. The patient was initially prescribed imatinib but demonstrated poor compliance. In February 2022, he presented to our outpatient department, reporting generalized weakness and fatigue. The BCR-ABL1 transcript level, measured by reverse transcription quantitative polymerase chain reaction, was 34.602%. Subsequently, dasatinib was initiated at a daily dose of 140 mg. After 1 month of therapy, he sought care at the emergency department with a history of two episodes of generalized tonic-clonic seizures. On examination, the patient was afebrile and had mild tachycardia, whereas his blood pressure remained normal. Neurological examination revealed bilateral grade 3 papilledema, but no limb weakness or signs of meningeal irritation were observed. Metabolic parameters, including renal and liver functions, were within normal limits. Serum sodium and potassium levels measured 139 mmol/L and 5.2 mmol/L, respectively. The serum calcium level was within the normal range (2.27 mmol/L). A complete blood count revealed hemoglobin level of 5.96 mmol/L, WBC counts of 4×109/L, and platelet count of 3×109/L. The NCCT head displayed mild hypodensity in the parieto-occipital region. Cerebrospinal fluid analysis indicated the presence of acellular cells, with cytological analysis showing no immature cells. MRI of the brain revealed T2/FLAIR hyperintensities in the left parietal and bilateral occipital white matter, with no evidence of diffusion restriction (Fig. 1). These findings were suggestive of PRES. Given the absence of any other apparent cause for this abnormality, dasatinib was considered as the likely culprit. The patient received levetiracetam as an anti-seizure treatment, and dasatinib was discontinued. He became seizure-free and showed complete symptomatic improvement 3 days after discontinuing the medication. Although EEG was planned, it was deferred for logistic reasons, and the patient did not experience any further seizures.
To the best of our knowledge, this case report is the first to describe atypical PRES from dasatinib use in a patient with CML. PRES is characterized by reversible subcortical vasogenic brain edema in patients with acute neurological symptoms (seizures, encephalopathy, headache, and visual disturbances). Clinical presentation of PRES is typically accompanied by elevated arterial pressure. Onset can be acute (within a few hr) or subacute (over days to wk). Encephalopathy, ranging from cognitive deficits to stupor and coma, is the predominant manifestation. Both focal and generalized seizures have been previously documented, with visual disturbances often occurring in cases involving the occipital lobe. Nonspecific neurological symptoms may include headache, nausea, and vomiting. Focal neurological deficits have been reported in approximately 10% of the cases [3].
The primary explanation for most PRES cases is the rapid development of hypertension that exceeds the upper limit of cerebral blood flow regulation, resulting in hyperperfusion. However, 15–20% of patients with PRES have normal or low blood pressure [4]. In such cases, cytokine activation leading to alteration in blood vessel permeability may also contribute to the pathophysiological changes associated with PRES.
MRI of the brain has identified various characteristic patterns of involvement in patients with PRES. Three primary descriptive variations exist in approximately two-thirds of patients: a dominant parieto-occipital pattern, a holohemispheric watershed pattern, and a superior frontal sulcus pattern. Brain edema can affect other regions, including the frontotemporal lobe (75%), cerebellum (50%), basal ganglia, and brainstem involvement (33%). Intracranial hemorrhage complicates 10–15% of cases [5].
Drug-induced PRES has been associated with the medications that target receptors involved in regulating vascular permeability or modulating the immune response [6]. Tyrosine kinase Inhibitor-associated PRES, although rare, is emerging as a serious adverse event related to treatment, as described in several case reports. Dasatinib, approved by the FDA for the treatment of adults with chronic, accelerated, myeloid- or lymphoid blast-phase CML and resistance or intolerance to prior therapy, including imatinib, is a potent, orally bioavailable inhibitor of several kinases, including BCR-ABL, SRC family kinases, c-KIT, and PDGFR-β [7]. Various adverse effects have been reported with dasatinib use including gastrointestinal, cardiovascular, endocrine, hematologic, and pulmonary toxicities. Vascular toxicity has been extensively studied, particularly in the context of pulmonary hypertension caused by this agent. Proposed mechanisms include vascular endothelial cell injury and apoptosis due to increased endoplasmic reticulum stress and reactive oxygen species production. Patients treated with dasatinib have shown increased circulating levels of markers associated with endothelial dysfunction [8]. Although we believe a similar mechanism may underlie alterations in cerebral circulation, leading to hyperperfusion and PRES, further studies are necessary to confirm this.
The treatment approach for PRES is symptom-focused and supportive. In cases with excessively elevated blood pressure, a 25% decrease in the MAP in the first several hours is typically recommended. Suspected drug-induced PRES necessitates discontinuation of the offending medication.
This case report represents the first documented instance of an unusual case of PRES induced by dasatinib therapy for CML. Following the termination of dasatinib treatment, clinical improvement in the symptoms was observed. Maintaining a high index of suspicion and promptly recognizing this entity are critical to preventing serious problems associated with the continued use of the offending agent.
REFERENCES
1. Fugate JE, Rabinstein AA. 2015; Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 14:914–25. DOI: 10.1016/S1474-4422(15)00111-8. PMID: 26184985.

2. McCormack PL, Keam SJ. 2011; Dasatinib: a review of its use in the treatment of chronic myeloid leukaemia and Philadelphia chromosome-positive acute lymphoblastic leukaemia. Drugs. 71:1771–95. DOI: 10.2165/11207580-000000000-00000. PMID: 21902298.
3. Fischer M, Schmutzhard E. 2017; Posterior reversible encephalopathy syndrome. J Neurol. 264:1608–16. DOI: 10.1007/s00415-016-8377-8. PMID: 28054130. PMCID: PMC5533845.

4. Rabinstein AA, Mandrekar J, Merrell R, Kozak OS, Durosaro O, Fugate JE. 2012; Blood pressure fluctuations in posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis. 21:254–8. DOI: 10.1016/j.jstrokecerebrovasdis.2011.03.011. PMID: 21536456.

5. Bartynski WS, Boardman JF. 2007; Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 28:1320–7. DOI: 10.3174/ajnr.A0549. PMID: 17698535. PMCID: PMC7977645.

6. Hobson EV, Craven I, Blank SC. 2012; Posterior reversible encephalopathy syndrome: a truly treatable neurologic illness. Perit Dial Int. 32:590–4. DOI: 10.3747/pdi.2012.00152. PMID: 23212858. PMCID: PMC3524908.

7. Nekoukar Z, Moghimi M, Salehifar E. 2021; A narrative review on adverse effects of dasatinib with a focus on pharmacotherapy of dasatinib-induced pulmonary toxicities. Blood Res. 56:229–42. DOI: 10.5045/br.2021.2021117. PMID: 34776414. PMCID: PMC8721448.

8. Guignabert C, Phan C, Seferian A, et al. 2016; Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 126:3207–18. DOI: 10.1172/JCI86249. PMID: 27482885. PMCID: PMC5004960.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download