TO THE EDITOR: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an important treatment for several hematologic malignancies but is associated with graft-versus-host disease (GVHD), which can lead to fatal outcomes [1]. Acute GVHD (aGVHD) can be treated with glucocorticoids. However, about half of the patients do not respond well. Patients who do not respond to steroids have a high mortality rate [2].
Gut microbiota is an important component of the intestinal immune system. In patients undergoing allo-HSCT, factors such as chemotherapy, radiation, altered nutritional patterns, and antibiotics lead to a significant reduction in microbiota diversity [3]. Studies have shown that reduced diversity is associated with poor outcomes, including aGVHD and relapse [4, 5]. Fecal microbiota transplantation (FMT) is increasingly being used to restore the gut microflora in patients with recurrent or refractory Clostridioides difficile infections [6]. It is also increasingly used to treat diseases associated with pathological microbiome imbalances, including inflammatory bowel disease, irritable bowel syndrome, and metabolic diseases [7, 8]. Therefore, the use of FMT to modulate intestinal microflora may be an alternative treatment option for steroid-refractory GVHD. Recently, efforts have been made to use fecal microbiota from healthy donors to treat intestinal GVHD [1, 9]. However, there is limited evidence supporting its effectiveness and the risk of infection transmission. This pilot study retrospectively evaluated the effectiveness and safety of frozen FMT in the treatment of aGVHD. Four patients underwent FMT for steroid-refractory GVHD in 2018 at Seoul St. Mary’s Hospital in Korea. A complete response (CR) was defined as the resolution of gastrointestinal symptoms or reduction of the steroid dose to 5 mg prednisone [10]. Clinical improvement was defined as a decrease in stool volume to <500 mL and resolution of bleeding [11]. Briefly, 375 mL of fecal microbiota from a healthy unrelated donor was sprayed into the patient’s cecum through colonoscopy or into the second portion of the duodenum through gastroscopy.
A 31-year-old female, diagnosed with severe aplastic anemia underwent haplo-HSCT from a familial mismatched donor (Table 1). The patient presented to our clinic with a skin rash and abnormal liver function on day +25 after haplo-HSCT. High-dose methylprednisolone (2 mg/kg) was initiated for treating skin and hepatic GVHD. The skin lesions did not improve despite high-dose steroid pulse treatment, and the patient developed melena after 5 days. On day +33 after haplo-HSCT, the patient was diagnosed with grade IV aGVHD, skin stage 4, liver stage 1, and gastrointestinal stage 3 according to a colonoscopic biopsy. Despite the initiation of high-dose steroids, there was no clinical response, and the bleeding progressively worsened. On day +36, FMT was discussed with the patient and informed consent was obtained. The patient received frozen fecal microbiota samples from an unrelated healthy donor. The first FMT did not affect the melena. Ten days after the FMT, Klebsiella pneumonia was identified in the blood culture. The patient received the second and third FMT doses after the sepsis improved. At the time of the second FMT, Escherichia coli bacteremia was present but resolved soon afterward. Bacteria cultured from the patients were not detected in the donor stool samples. Significant clinical improvement was observed, including a marked resolution of hematochezia and abdominal pain. Unfortunately, two weeks after the third FMT, ileus, and hematochezia recurred. Pseudomembranous colitis was confirmed by sigmoidoscopy. There was no clinical improvement despite medical treatment and a fourth FMT was performed, which led to partial remission on sigmoidoscopy on day +108 after haplo-HSCT (Fig. 1). The patient died of hepatic GVHD and septic shock on day +147.
A 52-year-old female underwent allo-HSCT from a matched-sibling donor for acute myeloid leukemia. The patient developed watery diarrhea, severe epigastric pain, and skin rash over the entire body on day +86 after allo-HSCT. She was diagnosed with grade III aGVHD, skin stage 2, liver stage 0, and GI stage 2, and high-dose methylprednisolone (1 mg/kg) was administered. Symptoms did not improve even after increasing the steroid dose to 2 mg/kg and adding mycophenolate mofetil. Ruxolitinib (5 mg, twice daily) was added, and the steroids were tapered. The volume and frequency of diarrhea decreased but the abdominal pain persisted. On day +140 after allo-HSCT, informed consent was obtained and FMT was performed. This led to significant improvement in GI symptoms, including bloating and epigastric pain. However, nausea and vomiting from ileus recurred 2 weeks after FMT. A second FMT was considered but was not performed because of persistent pancytopenia and hyperbilirubinemia. As hyperbilirubinemia progressed due to the exacerbation of hepatic GVHD, the patient’s condition deteriorated rapidly. The patient died of hepatic GVHD and sepsis on day +174 after allo-HSCT.
A 39-year-old female, diagnosed with multiple myeloma underwent allogeneic hematopoietic stem cell transplantation from a matched unrelated donor. The patient developed diarrhea, with a frequency >10 times a day, and spastic abdominal pain on day +30. Grade III aGVHD was diagnosed at skin stage 2, liver stage 0, and gastrointestinal stage 3. High-dose methylprednisolone (2 mg/kg) was added to the treatment regimen, which led to a decrease in the frequency of diarrhea to approximately five times a day but with no improvement in abdominal pain. On day +44 after the allo-HSCT, FMT was performed via colonoscopy. Three days after the FMT, the volume and frequency of diarrhea and abdominal pain improved. However, because diarrhea persisted after 3 weeks, a second FMT was performed. FMT was performed every 3–4 weeks depending on the patient’s medical condition. After the fourth FMT, ruxolitinib was added to the treatment regimen, and the gut GVHD remained in partial remission until the last follow-up (day +1,641).
A 48-year-old female, diagnosed with acute myeloid leukemia underwent allo-HSCT from a matched unrelated donor. The patient developed diarrhea at a frequency of >10 times a day and abdominal pain on day +18 after allo-HSCT. Grade IV aGVHD was diagnosed at skin stage 0, liver stage 0, and gastrointestinal stage 4. High-dose methylprednisolone (2 mg/kg) was initiated to treat aGVHD. On day +35 after allo-HSCT, FMT was performed after informed consent was obtained. Although the volume and frequency of the diarrhea improved, the patient developed abdominal distension and nausea. Three days after FMT, the patient developed a fever, and extended-spectrum beta-lactamase-negative E. coli and vancomycin-susceptible Enterococcus faecium were confirmed by blood culture. However, bacteria cultured in the patient’s blood were not identified in the donor feces. The patient was admitted to the intensive care unit for gastrointestinal septic shock 5 days after FMT. After recovery from shock, the patient underwent a colonoscopy for recurrent postprandial diarrhea. Although a partial response was observed endoscopically, symptoms of ileus, including abdominal distension, nausea, and vomiting, persisted. The patient’s general condition gradually deteriorated with persistent intestinal GVHD and newly diagnosed hepatic GVHD. The patient eventually died of active GVHD on day +94 after allo-HSCT.
Patients with steroid-refractory aGVHD have only approximately 50% and 20% 6-month and 2-year survival rates, respectively [12]. Despite the dismal prognosis, there are limited therapeutic options available for these patients. Allo-HSCT causes significant intestinal dysbiosis, which is associated with an increased GVHD risk [1]. FMT restores the beneficial functions of healthy microbiota by restoring normal fecal microbes in the gastrointestinal tract [1, 9]. However, the mechanism of action of FMT in the treatment of GVHD remains unclear. Healthy gut microbiota may produce metabolites with anti-inflammatory effects that can alleviate inflammation and the symptoms of GVHD. FMT may also be involved in the regulation of immune cells that mitigate systemic alloimmune response [9]. In this study, four patients with steroid-refractory aGVHD were treated with FMTs. The patients received conventional immunosuppressive treatments, including ruxolitinib in one case before FMT but did not respond. Three patients responded well to FMT, and treatment was repeated when symptoms recurred. One patient did not respond to FMT.
Only one of the four patients survived. The patient showed improvement in symptoms after each FMT; however, the symptoms recurred after a few days. After the fourth FMT, ruxolitinib was added and a partial response was achieved. Ruxolitinib is emerging as the best option for steroid-resistant GVHD, and studies on the combination of ruxolitinib and FMT are in progress [13, 14]. Further randomized clinical trials are required to determine whether the combination of FMT and ruxolitinib is an acceptable treatment option.
Many concerns remain regarding the safety of FMT for GVHD treatment. Two patients in this study also developed bacteremia following FMT but it is unclear whether FMT was the cause because the inoculum did not contain pathological bacteria. Metagenomic analyses of the donor material to track changes in the microflora after transplantation could not be performed in this study. A previous study showed that analysis of 16S ribosomal RNA from the gut microbiome can be used to elucidate its effect on patient outcomes after transplantation [15]. Future studies should focus on the molecular mechanisms using metabolomic analyses.
In summary, FMT shows promise for restoring microbial diversity and treating aGVHD in patients undergoing allo-HSCT. However, FMT remains experimental and the most effective route of administration, product formulation, volume, and frequency of the procedure has not yet been established. Further studies are required to evaluate the potential benefits of FMT in patients with steroid-refractory acute GVHD.
REFERENCES
1. Fredricks DN. 2019; The gut microbiota and graft-versus-host disease. J Clin Invest. 129:1808–17. DOI: 10.1172/JCI125797. PMID: 31042160. PMCID: PMC6486325.

2. Martin PJ, Rizzo JD, Wingard JR, et al. 2012; First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 18:1150–63. DOI: 10.1016/j.bbmt.2012.04.005. PMID: 22510384. PMCID: PMC3404151.

3. Holler E, Butzhammer P, Schmid K, et al. 2014; Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 20:640–5. DOI: 10.1016/j.bbmt.2014.01.030. PMID: 24492144. PMCID: PMC4973578.

4. Noor F, Kaysen A, Wilmes P, Schneider JG. 2019; The gut microbiota and hematopoietic stem cell transplantation: challenges and potentials. J Innate Immun. 11:405–15. DOI: 10.1159/000492943. PMID: 30286447. PMCID: PMC6738217.

5. Peled JU, Gomes ALC, Devlin SM, et al. 2020; Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 382:822–34. DOI: 10.1056/NEJMoa1900623. PMID: 32101664. PMCID: PMC7534690.
6. Juul FE, Garborg K, Bretthauer M, et al. 2018; Fecal microbiota transplantation for primary clostridium difficile infection. N Engl J Med. 378:2535–6. DOI: 10.1056/NEJMc1803103. PMID: 29860912.

7. Cui B, Feng Q, Wang H, et al. 2015; Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol. 30:51–8. DOI: 10.1111/jgh.12727. PMID: 25168749.
8. Ianiro G, Eusebi LH, Black CJ, Gasbarrini A, Cammarota G, Ford AC. 2019; Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 50:240–8. DOI: 10.1111/apt.15330. PMID: 31136009.

9. Qiao X, Biliński J, Wang L, et al. 2023; Safety and efficacy of fecal microbiota transplantation in the treatment of graft-versus-host disease. Bone Marrow Transplant. 58:10–9. DOI: 10.1038/s41409-022-01824-1. PMID: 36167905.

10. Ibrahim RB, Abidi MH, Cronin SM, et al. 2009; Nonabsorbable corticosteroids use in the treatment of gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 15:395–405. DOI: 10.1016/j.bbmt.2008.12.487. PMID: 19285626. PMCID: PMC3809114.

11. Castilla C, Pérez-Simón JA, Sanchez-Guijo FM, et al. 2006; Oral beclomethasone dipropionate for the treatment of gastrointestinal acute graft-versus-host disease (GVHD). Biol Blood Marrow Transplant. 12:936–41. DOI: 10.1016/j.bbmt.2006.05.010. PMID: 16920559.

12. Westin JR, Saliba RM, De Lima M, et al. 2011; Steroid-refractory acute GVHD: predictors and outcomes. Adv Hematol. 2011:601953. DOI: 10.1155/2011/601953. PMID: 22110505. PMCID: PMC3216266. PMID: 10fdb3c57b0c4114b28a6826d6ee477b.

13. Zeiser R, von Bubnoff N, Butler J, et al. 2020; Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 382:1800–10. DOI: 10.1056/NEJMoa1917635. PMID: 32320566.

14. Liu Y, Zhao Y, Qi J, et al. 2022; Fecal microbiota transplantation combined with ruxolitinib as a salvage treatment for intestinal steroid-refractory acute GVHD. Exp Hematol Oncol. 11:96. DOI: 10.1186/s40164-022-00350-6. PMID: 36352455. PMCID: PMC9644456. PMID: 48d75dcc279d441a982926ea135603dd.

15. Kusakabe S, Fukushima K, Maeda T, et al. 2020; Pre- and post-serial metagenomic analysis of gut microbiota as a prognostic factor in patients undergoing haematopoietic stem cell transplantation. Br J Haematol. 188:438–49. DOI: 10.1111/bjh.16205. PMID: 31566729.

Fig. 1
Endoscopic response after fecal microbiota transplantation. (A) Patient 1, at time of FMT. (B) Patient 1, on day +108. (C) Patient 2, at time of FMT. (D) Patient 2, on day +35. (E) Patient 3, at time of FMT. (F) Patient 3, on day +201. (G) Patient 4, at time of FMT. (H) Patient 4, on day +45.
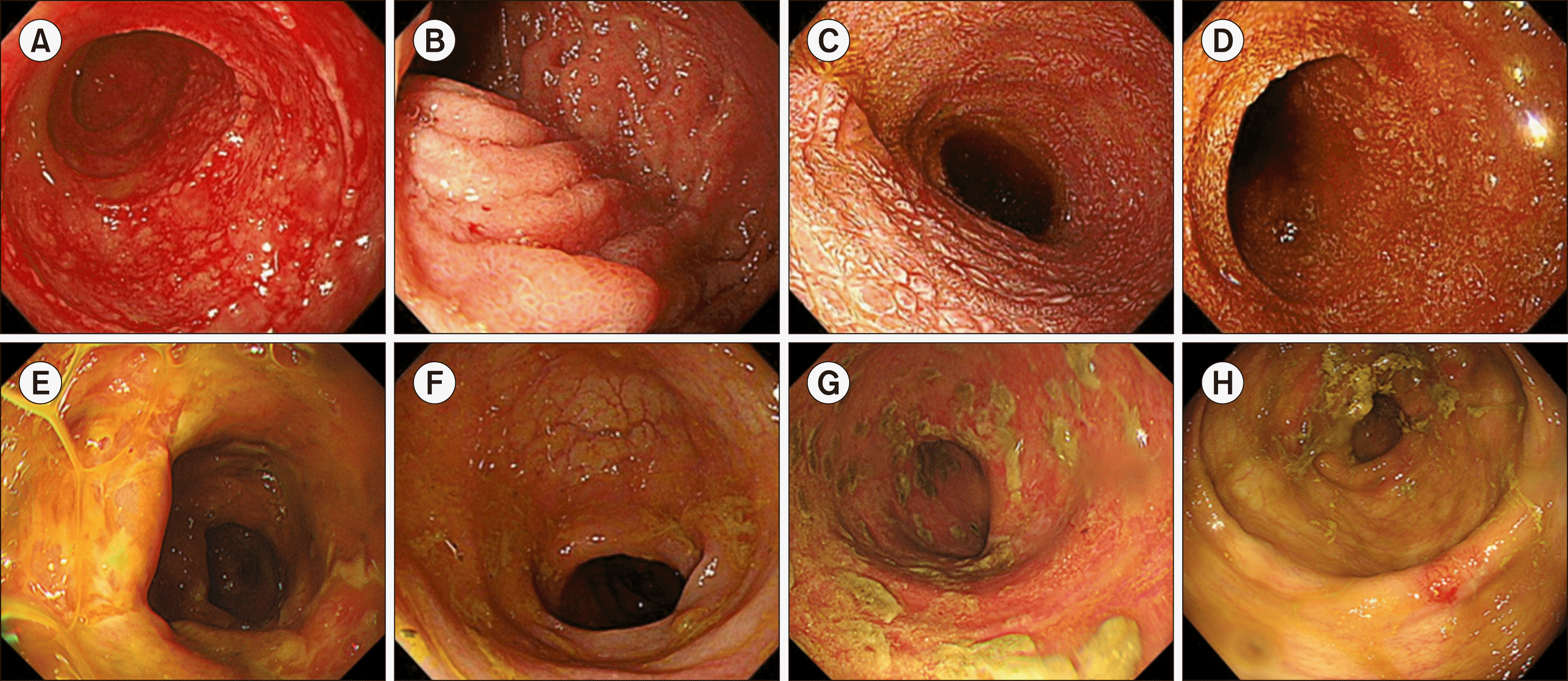
Table 1
Patient characteristics, adverse events, and responses.
Abbreviations: aGVHD, acute graft-versus-host disease; AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; C. difficile, Clostridioides difficile; CpS, cyclophosphamide; CsA, cyclosporine A; FK, tacrolimus; FMT, fecal microbiota transplantation; HSCT, hematopoietic stem cell transplantation; MM, multiple myeloma; MMF, mycophenolate mofetil; MSD, matched sibling donor; MTX, methotrexate; MUD, matched unrelated donor; TBI, total body irradiation; VSAA, very severe aplastic anemia.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download