Abstract
Purpose
The da Vinci single-port (SP) system has been used in various surgical fields, including colorectal surgery. However, limited experience has been reported on its safety and feasibility. This study aims to evaluate the short-term outcomes of SP robotic surgery for the treatment of rectal cancer compared with multiport (MP) robotic surgery.
Methods
Rectal cancer patients who underwent curative resection in 2020 were reviewed. A total of 43 patients underwent robotic total mesorectal excision (TME), of which 26 (13 in each group, SPTME
vs. MPTME) were included in the case-matched cohort for analysis. Intraoperative and postoperative outcomes and pathological results were compared between the 2 groups.
Results
Median tumor height was similar between the 2 groups (SPTME
vs. MPTME: 5.9 cm [range, 2.2–9.6 cm] vs. 6.7 cm [range, 3.4–10.0 cm], P = 0.578). Preoperative chemoradiotherapy was equally performed (38.5%). The median estimated blood loss was less (20.0 mL [range, 5.0–20.0 mL] vs. 30.0 mL [range, 20.0–30.0 mL], P = 0.020) and the median hospital stay was shorter (7 days [range, 6–8 days] vs. 8 days [range, 7–9 days], P = 0.055) in the SPTME group. Postoperative complications did not differ (SPTME
vs. MPTME: 7.7% vs. 23.1%, P = 0.587). One patient in the SPTME group and 3 in the MPTME group experienced anastomotic leakage.
The newly developed da Vinci single-port (SP) system (Intuitive Surgical) has been increasingly used in various surgical fields [123]. Although it was first established for performing precise procedures in a narrow area, the advantages of single-site surgery have encouraged colorectal surgeons to use it for colorectal cancer surgery. Unfortunately, only a few case series have been reported [456789] and more studies supporting its use in complex procedures, such as rectal cancer surgery, are needed.
The multiport (MP) da Vinci Xi system (Intuitive Surgical), the previous version of the SP system, is optimal for performing total mesorectal excision (TME) based on advanced technologies, including three-dimensional magnified vision, articulated instruments, or better ergonomics [1011]. The SP robotic system basically has the same advantages as the MP robotic system and improves some limitations of the previous MP robot [12]. The SP system has reduced the difficulties associated with trocar position and docking. Additionally, the 360° multi-quadrant anatomical access and single-arm design mitigate the internal and external collisions of the robotic arms, while the additional joints of the SP instruments help the operator manipulate the instruments with less restriction arising from collisions of the robotic arms. The current SP system, however, also has some shortcomings such that it does not include a vessel sealer, suction, or stapler. Eventually, all these procedures could be performed with great help from a bedside assistant. In addition, some surgeons still doubt whether the SP instruments have enough power for precise rectal dissection in a limited pelvic cavity. A comparison study of the SP system with a previous robot system has not yet been conducted to demonstrate whether the SP robot can be a viable option for performing rectal cancer surgery. Hence, in this study, we aimed to evaluate the safety and feasibility of the SP robotic system by comparing it with previous MP robotic systems for the treatment of rectal cancer.
This study was performed in line with the principles of the Declaration of Helsinki. All patients received an extensive explanation of the procedure and provided written informed consent for the publication of this report including all clinical images. This study was approved by the Institutional Review Board of Kyungpook National University Chilgok Hospital (No. 2020-08-026).
Rectal cancer patients who underwent curative resection using the robotic approach at Kyungpook National University Chilgok Hospital in 2020 were retrospectively reviewed. Patients who underwent abdominoperineal resection, combined resections including liver, lung, and synchronous colon cancer, and salvage operations for recurrences were excluded. Demographic characteristics, intraoperative outcomes including the operative time of each step and estimated blood loss, postoperative outcomes including hospital stay and complications, and pathologic results were retrospectively reviewed. Patients who underwent MP robotic TME were case matched for age, sex, tumor location, and preoperative treatment with patients who underwent SP robotic TME (1:1).
Preoperative workup included tumor markers, digital rectal examination, colonoscopy, abdominopelvic CT, and pelvic magnetic resonance imaging. Patients with T3, T4, or node-positive disease received long-course (50 Gy in 25 fractions for 5 weeks, 5-fluorouracil–based chemotherapy) or short-course (25 Gy in 5 fractions for 5 days) preoperative chemoradiotherapy (CRT). Radical surgery was performed 6–8 weeks after the completion of radiotherapy. The operative approach was decided in joint meetings, which included the surgeon, patients, and their physicians.
Four surgeons performed the operation. The da Vinci Xi system and da Vinci SP system were used for MP and SP robotic TME procedures, respectively.
The surgical steps were identical, excluding port numbers and positions. The detailed surgical techniques for SP or MP robotic TME have been described in previous studies [111213]. The patient was tilted to the right and placed in the Trendelenburg position. MP robotic TME has been described previously [11]. Briefly, the procedures involved a 12-mm supraumbilical port for the camera and 3 robotic working ports of 8 mm. One or 2 additional laparoscopic trocars were introduced for traction and operation; they were placed in an oblique linear line from the anterior superior iliac spine to the right upper quadrant. We preferred a hybrid technique consisting of laparoscopy for colon and splenic flexure (SF) mobilization, robot-assisted inferior mesenteric artery (IMA) ligation, and proctectomy with TME.
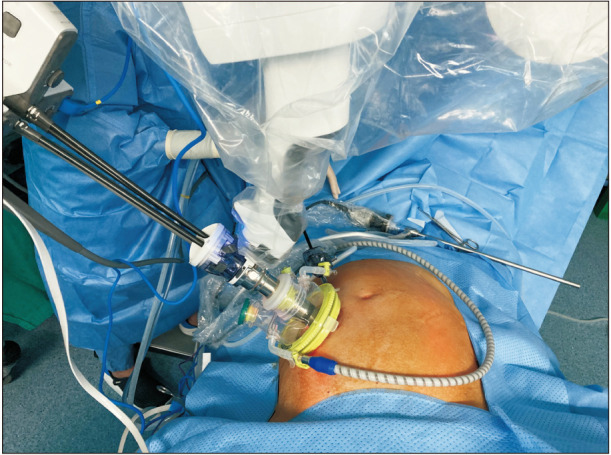
For the SP robotic TME, a single 4-cm transverse incision was made in the right lower quadrant, and Uniport (Dalim) containing a 25-mm multichannel SP trocar, 12-mm assistant port, and 2 assistant ports in 5 mm were introduced (Fig. 1) [12]. The SP trocar was composed of 4 lumens for the camera, an SP round tooth retractor, SP monopolar curved scissors, and fenestrated bipolar forceps. The assistant used other ports for suction and retraction. In addition, a 5-mm laparoscopic assistant port was placed 10 cm cephalic to the SP trocar. After robotic docking, high ligation of the inferior mesenteric vessels with complete lymph node dissection and total mobilization of the left colon were performed using an entirely robotic approach.
After complete dissection of the rectum, it was transected using a laparoscopic endolinear stapler through the 12-mm port in the Uniport. The specimen was extracted through a 4-cm minilaparotomy. Intracorporeal end-to-end anastomosis was created using a circular stapler. Intersphincteric resection and hand-sewn side-to-end coloanal anastomosis were performed in patients with very low tumors. A pelvic drain was placed, and a protective ileostomy was created in the right lower quadrant with the same incision at the surgeon’s discretion.
All statistical analyses were performed using IBM SPSS Statistics ver. 26.0 (IBM Corp.). Quantitative variables following normal distribution were reported as mean ± standard deviation. Qualitative variables were reported as the number and percentages of cases. Continuous variables were tested for normality (Shapiro-Wilk normality test). The 2 groups were compared using 2-sampled Student t-tests or Kruskal-Wallis rank-sum tests. Categorical variables were assessed using the chi-square test or Fisher exact test. Significance was set at a two-sided P-value of <0.05.
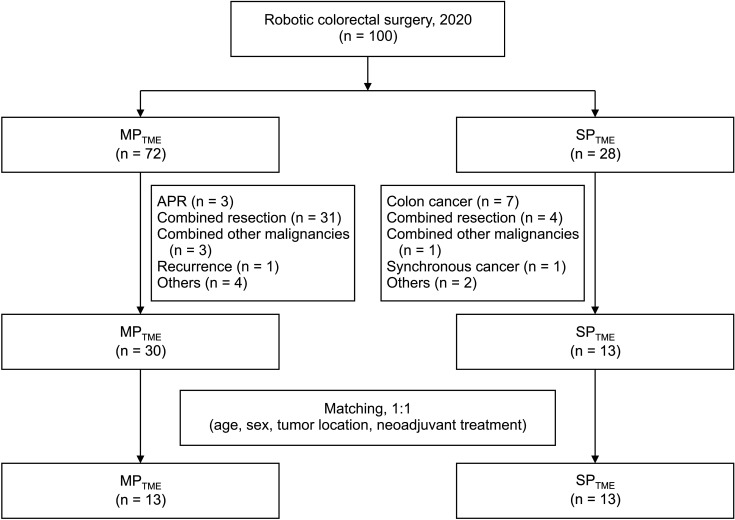
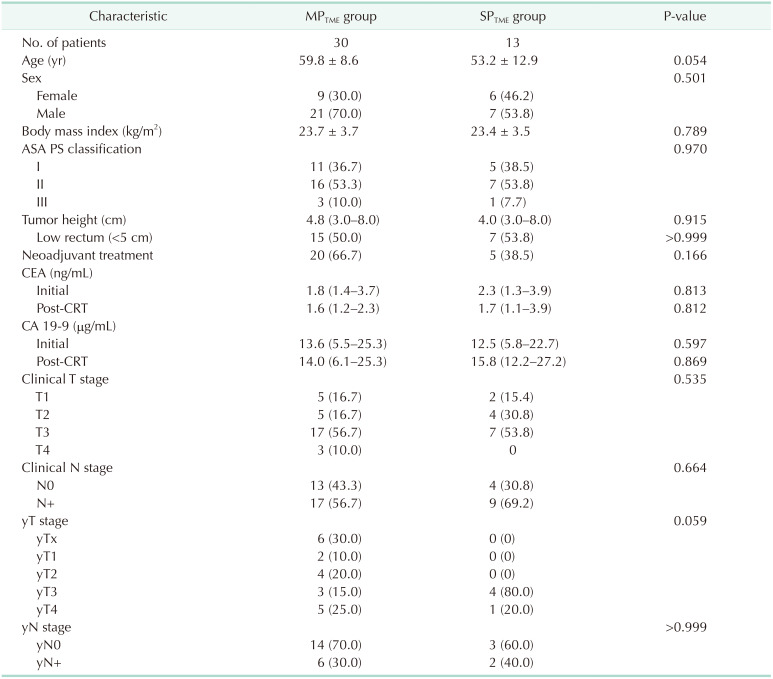
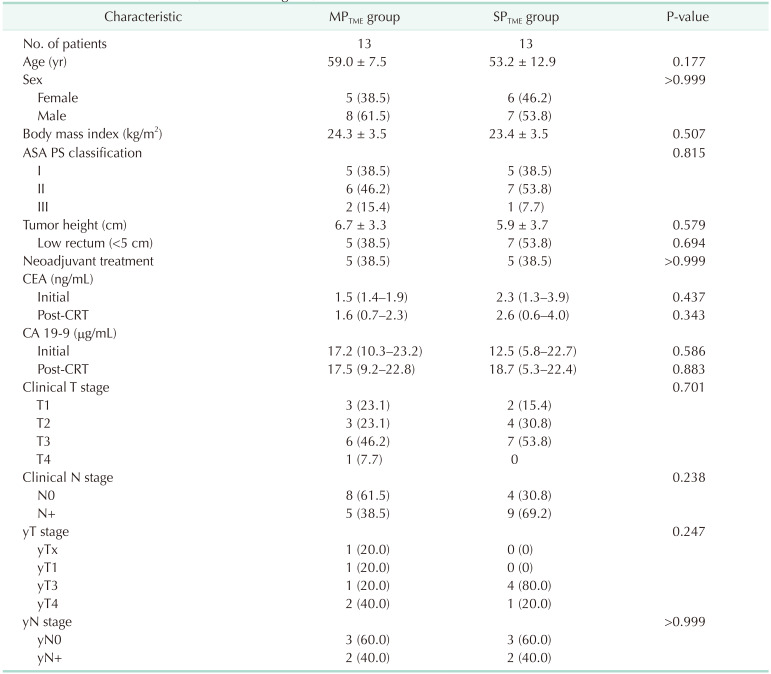
A total of 100 patients who underwent robotic TME in 2020 were identified. After exclusion which included abdominoperineal resection, combined resections including liver, lung, synchronous colon cancer and even distant lymph node dissection, and operations for recurrences, 43 patients with rectal cancer were finally evaluated. Most cases of combined resection included lateral pelvic lymph node dissection for 24 patients and 3 patients for paraaortic lymph node dissection. Benign diseases or previous malignancy history were also excluded. Of the 43 patients, 30 underwent MP robotic surgery, and the remaining 13 underwent SP robotic surgery (Fig. 2). The initial 2 groups showed significant differences in clinical characteristics; the MPTME group had more male patients, a higher rate of preoperative CRT, and a greater number of higher clinical T staging cases (Table 1). Thirteen patients from both groups were selected after 1:1 case matching by age, sex, tumor location, and preoperative treatment. The median tumor height from the anal verge was similar between the 2 groups (SPTME
vs. MPTME: 5.9 cm [range, 2.2–9.6 cm] vs. 6.7 cm [range, 3.4–10.0 cm], P = 0.579). In addition, preoperative CRT was performed equally in both groups (38.5%) (Table 2).
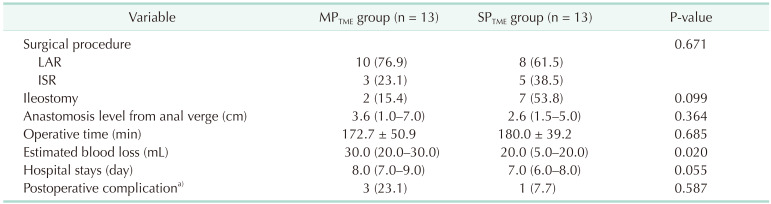
The perioperative outcomes are presented in Table 3. The levels of anastomosis from the anal verge were similar in the 2 groups (2.6 cm [range, 1.5–5.0 cm] vs. 3.6 cm [range, 1.0–7.0 cm], P = 0.364). The median total operative times were similar in the 2 groups (180 minutes [range, 122–224 minutes] vs. 172 minutes [range, 141–219 minutes], P = 0.685). The median estimated blood loss was significantly lower in the SPTME group (20.0 mL [range, 5.0–20.0 mL] vs. 30.0 mL [range, 20.0–30.0 mL], P = 0.020). The median hospital stay was shorter in the SPTME group (7 days [range, 6–8 days] vs. 8 days [range, 7–9 days], P = 0.055), and postoperative complications did not differ between the 2 groups (SPTME
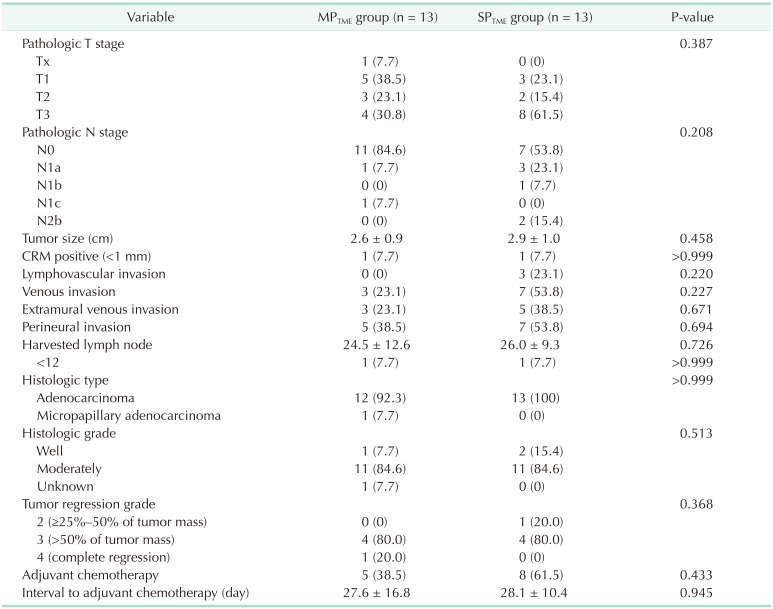
vs. MPTME: 7.7% vs. 23.1%, respectively; P = 0.587). A total of 4 patients (3 in the MPTME group and 1 in the SPTME group) had postoperative complications; all had anastomotic leakage. Three of them had ileostomy creation surgery, and one underwent redo anastomosis for ischemia. The pathological findings are presented in Table 4; no significant differences between the 2 groups. The circumferential resection margin measured less than 1 mm in 1 patient from each group.
Our study was the first case-matched study of SP and MP systems for TME to treat rectal cancer. The SPTME group showed comparable perioperative and pathological outcomes to the MPTME group. In addition, the SPTME group had less estimated blood loss and a shorter postoperative hospital stay. Although further studies are needed to confirm the study outcomes based on a large cohort, the SP robotic system may be considered one option for the treatment of rectal cancer.
In this study, we included the first cases of SP robotic surgery. Therefore, a discrepancy was observed in the preoperative CRT rate, men and women predominance rates, clinical T stage, and tumor location because we first adopted the SP system in patients with relatively early rectal cancer located in the midrectum. However, we have tried to expand the indication of SP surgery to a more advanced one in the later phase. Therefore, we needed to conduct a case-matched study for an appropriate comparison between the 2 robotic systems.
Other procedures between the 2 groups were the same; however, in the SPTME group, all procedures were performed using a robotic approach, including IMA ligation and SF mobilization. Whereas, in the MPTME group, a hybrid technique consisting of conventional laparoscopy and robotic surgery was preferred [11]. However, the 2 groups had no significant difference in operating time. By interpreting these results, one might conclude that the SP system took no longer to perform these procedures than the MP system. The 360° rotating system of the SP robot can easily move all instruments, including the camera, to another place in the abdomen, and the time to redock the robot or add trocars can be reduced. This is potentially advantageous when performing a multi-quadrant operation.
Several studies have shown that SP surgery is safe and feasible. Luján et al. [5] demonstrated that there were no significant differences in perioperative outcomes between SP and MP laparoscopic colectomy through the systematic review and meta-analysis of more than 2,800 procedures. Another study which included 36 patients who underwent single plus one-port robotic surgery and 61 patients with MP laparoscopic surgery for left-sided colon cancer showed that the clinicopathologic outcomes were comparable, but the cosmetic outcomes of SP surgery were superior to those of MP surgery [14]. In the SIMPLE study, there were no significant differences in the 30-day postoperative complication rate and all other outcomes between SP and MP laparoscopic surgery for colon cancer. However, 1.7% of patients required conversion to open surgery, and 15% required more ports in the SP laparoscopic surgery group. The main causes of conversion are adhesions and difficult locations of tumors or dissection [4]. Several other causes interrupt single-incision procedures, such as achieving traction or creating fields, indicating that the SP procedure is challenging in the laparoscopic or even robotic system. In addition, the SIMPLE study was conducted on patients with colon cancer, and no trials have yet evaluated rectal cancer treatment using an SP procedure. Based on the technical advancements of the SP robot system and the skills of experts, we were able to accomplish SP robotic surgery for rectal cancer despite these difficulties. Previous studies also demonstrated that a single-incision surgery might have better results in terms of pain scores, recovery times, and cosmetics when compared with MP surgery, which concluded that the SP system is a safer alternative to the MP system in colorectal surgery [151617].
A new SP system has been developed to overcome some limitations of previous robots. There were technical difficulties in terms of docking and resistance arising from collisions of moving instruments. However, in the new SP robot, docking time has been shortened due to the use of a single incision, single-access port, and single docking. In addition, each robotic instrument has its own additional elbow joint proximal to the wrist joint, which can move more freely, and the operator can visualize the flexion and extension of each instrument using the camera placed in the single robotic arm. The camera also has joints that can articulate independently, and various camera modes including below or above, allow surgeons to visualize deeper operative fields while reducing collisions with other instruments. Having sight in a deep pelvis can accelerate safer performance by the surgeon.
Although the current SP system has its advantages, a few significant problems still need to be addressed. Since the system lacks advanced instruments such as a vessel sealer, suction, or a stapler, the assistant would need to perform those jobs using the laparoscopic assistant port or using other trocars sharing the Uniport. Moreover, in imminent situations, such as bleeding, limited instrumentation may lead to severe problems such as massive bleeding or open conversion. Since the SP system is focused on a close rather than a distant view, proper suction is essential for keeping a clear viewing field. SP robotic instruments need to be developed. In addition, the elbow joint of each instrument, which had previously been an advantage, could not be bent in a deeper, narrower cavity. The operator should dissect using only the movement of the wrist joint. However, the longer distance between the 2 joints tends to interfere with precise dissection. Another problem is that the patient group selection is still limited, including a lack of data on patients with obesity or advanced rectal cancer. Although the sufficient force of each SP robotic instrument was demonstrated in various operations, it might be still doubtful in difficult cases such as obese or advanced rectal cancer patients. It is necessary to find an appropriate indication for the SP system through accumulated data by further application in patients with these difficult rectal cancers.
In our study, there was one case of postoperative complications in the SPTME group. The patient had a fever of 38℃ and hematochezia, which were diagnosed due to congestive ischemia of the neorectum. He underwent laparoscopic redo anastomosis, which required only an additional 5-mm port. The patient was discharged without further complications. These complications may have been related to the anastomosis rather than the new SP robotic technique. There were no significant differences in the pathologic outcomes between the 2 groups.
To our knowledge, this study was the first to evaluate the safety and feasibility of the SP robotic system by comparing it with previous MP robotic systems to treat rectal cancer. However, this study had several limitations. Firstly, it included a small number of patients. A larger-scale analysis, including more patients, is needed to support our conclusion. Including additional data beyond 2020 might strengthen the study’s findings and consider the potential impact of any changes in surgical practices or advancements in technology during subsequent years. Secondly, oncologic safety following long-term follow-up is important to address the safety of the procedure, but this study only focused on short-term outcomes. Third, as it was a retrospective study, selection bias was inevitable. To mitigate this bias, we conducted 1:1 matching. In circumstances of lack of experience, it may have the power to compare 2 different approaches. Fourth, there were no definite comparisons of pain or cosmetic scores in this study. A detailed score comparison of pain and cosmetic satisfaction is required to substantiate the benefits of the SP system. In addition, no defined indications for SP robotic surgery have been established. Thus, in the early stages of SP use, surgery was mainly performed for early and small rectal cancers. Based on our accumulated experience, we believe an accurate indication for SPTME can be made. Further prospective randomized controlled trials based on large cohorts are warranted to confirm these results further.
In conclusion, our study has shown similar perioperative outcomes between the SP and MP robotic systems for TME procedures, and we believe that the SP robotic system can be considered a viable surgical option for the treatment of rectal cancer. Further prospective randomized trials based on larger cohorts are required to support our findings.
References
1. Jones R, Dobbs RW, Halgrimson WR, Vigneswaran HT, Madueke I, Wilson J, et al. Single port robotic radical prostatectomy with the da Vinci SP platform: a step by step approach. Can J Urol. 2020; 27:10263–10269. PMID: 32544051.
2. Van Abel KM, Yin LX, Price DL, Janus JR, Kasperbauer JL, Moore EJ. One-year outcomes for da Vinci single port robot for transoral robotic surgery. Head Neck. 2020; 42:2077–2087. PMID: 32190942.

3. Park HS, Lee J, Lee H, Lee K, Song SY, Toesca A. Development of robotic mastectomy using a single-port surgical robot system. J Breast Cancer. 2020; 23:107–112. PMID: 32140275.

4. Lee YS, Kim JH, Kim HJ, Lee SC, Kang BM, Kim CW, et al. Short-term outcomes of single-port versus multiport laparoscopic surgery for colon cancer: the SIMPLE multicenter randomized clinical trial. Ann Surg. 2021; 273:217–223. PMID: 32209897.

5. Luján JA, Soriano MT, Abrisqueta J, Pérez D, Parrilla P. Single-port colectomy vs multi-port laparoscopic colectomy: systematic review and meta-analysis of more than 2800 procedures. Cir Esp. 2015; 93:307–319. PMID: 25687624.

6. Marks JH, Salem JF, Anderson BK, Josse JM, Schoonyoung HP. Single-port robotic left colectomy: first clinical experience using the SP robot (rSILS). Tech Coloproctol. 2020; 24:57–63. PMID: 31832798.

7. Marks JH, Kunkel E, Salem J, Martin C, Schoonyoung HP, Agarwal S. rSILS: initial clinical experience with single-port robotic (SPr) right colectomy. Tech Coloproctol. 2020; 24:817–822. PMID: 32451805.

8. Kneist W, Stein H, Rheinwald M. Da Vinci single-port robot-assisted transanal mesorectal excision: a promising preclinical experience. Surg Endosc. 2020; 34:3232–3235. PMID: 32394173.

9. Choi MS, Yun SH, Oh CK, Shin JK, Park YA, Huh JW, et al. Learning curve for single-port robot-assisted rectal cancer surgery. Ann Surg Treat Res. 2022; 102:159–166. PMID: 35317355.

10. Ryu HS, Kim J. Current status and role of robotic approach in patients with low-lying rectal cancer. Ann Surg Treat Res. 2022; 103:1–11. PMID: 35919115.

11. Park JS, Choi GS, Lim KH, Jang YS, Jun SH. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol. 2010; 17:3195–3202. PMID: 20589436.

12. Kim HJ, Choi GS, Song SH, Park JS, Park SY, Lee SM, et al. An initial experience with a novel technique of single-port robotic resection for rectal cancer. Tech Coloproctol. 2021; 25:857–864. PMID: 34052901.

13. Kim HJ, Choi GS, Park JS, Park SY. Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon’s experience. Dis Colon Rectum. 2014; 57:1066–1074. PMID: 25101602.

14. Bae SU, Jegon WK, Baek SK. Single plus one-port robotic surgery using the da Vinci single-site platform versus conventional multi-port laparoscopic surgery for left-sided colon cancer. Wideochir Inne Tech Maloinwazyjne. 2022; 17:179–187. PMID: 35251404.

15. Marks JH, Montenegro GA, Shields MV, Frenkel JL, Marks GJ. Single-port laparoscopic colorectal surgery shows equivalent or better outcomes to standard laparoscopic surgery: results of a 190-patient, 7-criterion case-match study. Surg Endosc. 2015; 29:1492–1499. PMID: 25277473.

16. Kang BM, Lee YS, Kim JH, Kim HJ, Lee SC, Kim CW, et al. Quality of life and patient satisfaction after single- and multiport laparoscopic surgery in colon cancer: a multicentre randomised controlled trial (SIMPLE Trial). Surg Endosc. 2021; 35:6278–6290. PMID: 33141277.

17. Jung KU, Yun SH, Cho YB, Kim HC, Lee WY, Chun HK. Single incision and reduced port laparoscopic low anterior resection for rectal cancer: initial experience in 96 cases. ANZ J Surg. 2016; 86:403–407. PMID: 25041064.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download