Abstract
Purpose
Perioperative transfusion is reported to be an independent risk factor not only for postoperative complications but also for early recurrence of periampullary carcinoma after pancreaticoduodenectomy (PD). The purpose of this study was to evaluate the safety and efficacy of ferric carboxymaltose (FCM) in reducing the need for perioperative transfusion in iron deficiency anemia patients scheduled for PD.
Methods
Twenty-two male patients (hemoglobin [Hb] 7 to <13 g/dL) and 18 female patients (Hb 7 to <12 g/dL) were enrolled in the study group and administered FCM 1–3 weeks before PD. The perioperative transfusion rate was the primary endpoint; morbidity, length of postoperative hospital stay, change in hematological parameters after FCM injection, and adverse effects of FCM were also investigated.
Results
The perioperative transfusion rate of the study group was 22.5% (9 of 40). Hb level was significantly higher on the day of the operation compared to baseline (P < 0.001). Levels of Hb, transferrin saturation, and ferritin were higher at the follow-up compared to baseline (P = 0.008, P = 0.033, and P < 0.001, respectively).
Pancreaticoduodenectomy (PD) is the only treatment modality with realistic potential for improving the long-term survival of patients with periampullary carcinoma. Despite refinement of surgical techniques, PD is still associated with significant bleeding and the requirement for transfusion, because of the anatomic complexity and long operation time. Postoperative hemorrhage is one of the most serious complications of PD [123]. The perioperative transfusion rate has decreased but remains high at up to 60% [456].
Allogeneic RBC transfusion during the perioperative period is associated with increased morbidity and mortality [78]. Perioperative transfusion has also been reported to lead to adverse oncologic outcomes, such as early tumor recurrence and decreased overall survival [91011]. Blood transfusion was reported to induce host immunosuppression via several mechanisms, including transfusion-induced suppression of natural killer cell and monocyte phagocytic activity, increased suppressor T-cell activity (resulting in inhibition of interleukin-2 production), and concomitant soluble Fas ligand and human leukocyte antigen-molecule transfusion [12131415]. Recent data also suggested that transfusion timing may be critical [6]. We previously reported that the perioperative RBC transfusion rate was 46% among 244 PD patients. Moreover, perioperative RBC and intraoperative transfusion were associated with decreased overall survival in patients who underwent PD for periampullary cancer [11]. Also, preoperative hemoglobin (Hb) of <12 mg/dL was an independent predictor of perioperative transfusion, along with female sex, a long operation time, and portal vein resection [11]. In that study, 49% (120 of 244) of PD patients were anemic (Hb of <12 mg/dL); most of the cases were of iron deficiency anemia (IDA) due to lack of iron intake, inability to absorb iron or chronic bleeding, etc.
Recent studies reported that infusion of intravenous iron in patients with postoperative anemia rapidly improved the levels of Hb, serum ferritin, and transferrin saturation (TSAT), and also prevented indiscriminate transfusion [161718]. In another recent study, ferric carboxymaltose (FCM) administered preoperatively in elderly patients with colorectal cancer influenced reducing blood transfusion and intensive care unit admissions after the operation [19]. A single large infusion of FCM has been reported to exert its effects more rapidly, and to lead to fewer gastrointestinal adverse effects, than an oral iron supplement [20]. It also raised Hb levels higher and caused less anemia just before surgery [2122]. Furthermore, Kim et al. [18], working in the Center for Gastric Cancer at our institute, reported that FCM was effective in patients with acute isovolemic anemia following gastrectomy.
Perioperative RBC transfusion is oncologically unsafe and preoperative anemia is significantly associated with perioperative transfusion in patients undergoing PD for periampullary cancer; thus, we believe that reducing perioperative RBC transfusion by increasing preoperative Hb in anemic patients could be very valuable for patient management. We posited that FCM could be effective for reducing perioperative RBC transfusion in patients with IDA scheduled for PD, and tested this hypothesis in the present study.
This was a prospective, single-center, single-arm phase II trial. The study was approved by the Institutional Review Board of the National Cancer Center, Korea (No. NCCCTS-13-709) and registered on ClinicalTrial.gov (No. NCT02628860). Written informed consent was obtained from all patients. The FCM protocol was based on a previous randomized clinical trial of patients who underwent gastrectomy at our institute [1823].
Patients were enrolled between October 2014 and December 2018. The inclusion criteria were as follows: aged >19 years, scheduled for PD for periampullary cancer, preoperative Hb level of 7–12.9 g/dL in males and 7–11.9 g/dL in females, mean corpuscular volume (MCV) of ≥ 95 µm3, and TSAT of ≥35%.
Patients with Hb levels of <7 g/dL needed preoperative transfusions and were excluded from the study. The diagnosis of IDA can be difficult; we took an MCV of ≥95 µm3 and TSAT of ≥35% to indicate IDA. The common criteria of IDA are as follows: microcytic RBC; serum ferritin, <30 ng/mL; and TSAT index, <20%. Although a low level of serum ferritin is a valuable parameter for diagnosing IDA, ferritin can be elevated even in IDA patients with hyperbilirubinemia or acute/chronic inflammation.
The exclusion criteria were as follows: concurrent medical conditions that would prevent compliance or jeopardize the health of the patient; hypersensitivity to any component of the treatment; current severe infection or inflammation; history of transfusion and erythropoietin or intravenous iron infusion of >500 mg within the 4 weeks before screening; MCV of >95 µm3 or TSAT of >35%; preoperative Hb of <7 g/dL; pregnancy or lactation; decreased renal function (defined as creatinine clearance of <50 mL/min/1.73 m2, calculated based on the estimated glomerular filtration rate); and chronic liver disease or increase in liver enzymes (aspartate aminotransferase and alanine aminotransferase) to >5 times the upper limit of normal.
FCM (Ferinject, Vifor Pharma Pty Ltd.) was administered as an intravenous infusion; 1,000 mg of FCM was mixed with 250 mL of normal saline for patients with a body weight of ≥50 kg, while 500 mg of FCM was mixed with 100 mL of normal saline for those with a body weight of <50 kg (1–3 weeks before PD). Perioperative RBC transfusion was performed at the surgeon’s discretion if the Hb level was <7 g/dL or vital signs became unstable.
This study evaluated the safety and efficacy of FCM for reducing perioperative transfusion in IDA patients scheduled for PD. The primary endpoints were the perioperative and postoperative (within 7 days) transfusion rates. The secondary endpoints were postoperative complications, length of hospital stay after PD, changes in hematological parameters (Hb, ferritin, and TSAT) after FCM injection, and adverse effects of FCM which were based on the previous literature [21]. Hematological parameters were measured at the time of enrollment, during the operation, and 4–6 weeks after PD. Adverse events were also noted.
Among 125 patients who underwent PD at our institute between January 2012 and September 2013, the perioperative transfusion rate was 30.4% (38 of 125). The transfusion rate for the patients with preoperative Hb levels of 7–11.9 g/dL was 44.2% (23 of 52), and for the patients with Hb of ≥12g/dL was 20.5% (15 of 73). We hypothesized that preoperative Hb levels would increase following FCM injection, and that the transfusion rate of patients with preoperative Hb levels of 7–11.9 g/dL (44.0%) would decrease to 23.0%. A sample size of 43 had 90% power to detect a 21.0% decrease (from 44.0% to 23.0%) in the perioperative transfusion rate using a 1-sided binomial test at a significance level of 5%. Assuming a 10% dropout rate, 48 samples were needed.
In the study group, Hb levels were analyzed using the paired t-test, and ferritin and TSAT levels using the Wilcoxon signed-rank test, at baseline, on the day of the operation, and at the 4–6-week follow-up. We compared the characteristics of the study and historical control groups. The control group consisted of 54 patients who underwent PD between January 2012 and September 2013. The same Hb inclusion criteria were used as in the study group (preoperative Hb of 7 to <13 g/dL in males and 7 to <12 g/dL in females). A paired t-test was used to analyze continuous variables, while the Wilcoxon signed-rank test was used to analyze continuous variables that did not follow a normal distribution, as well as categorical variables. Perioperative transfusion rates were compared between the study and control groups using logistic regression after adjusting for the estimated blood loss (EBL).
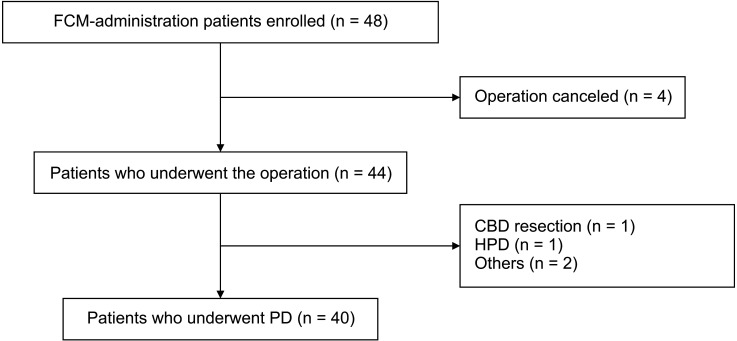
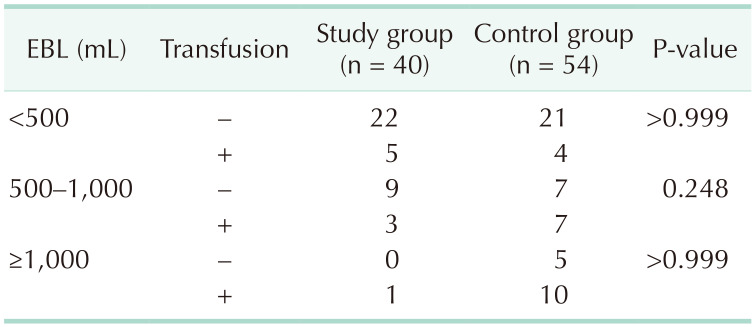
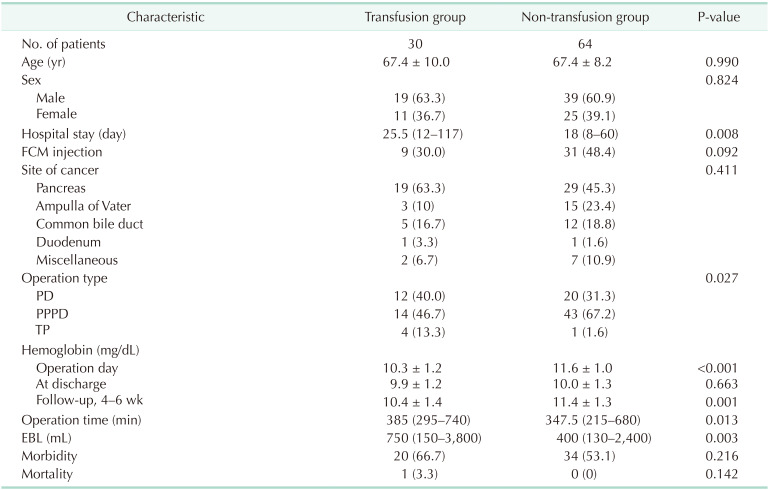
Between October 2014 and December 2018, 8 of 48 enrolled patients dropped out of the study; thus, 40 patients were included in the final analysis (Fig. 1), of whom 22 were male and 18 were female. The mean age of the patients was 69.1 ± 7.9 years. The median time from FCM administration to PD was 8 days (range, 7–33 days); 1,000 mg of FCM was administered to 32 patients and 500 mg to 8 patients. The median hospital stay after PD was 28 days (range, 11–60 days). Fourteen patients underwent standard PD and 25 had pylorus-preserving PD. One patient underwent a total pancreatectomy. The median operation time was 352.5 minutes (range, 245–680 minutes) and the median amount of EBL was 400 mL (range, 130–3,300 mL) (Table 1).
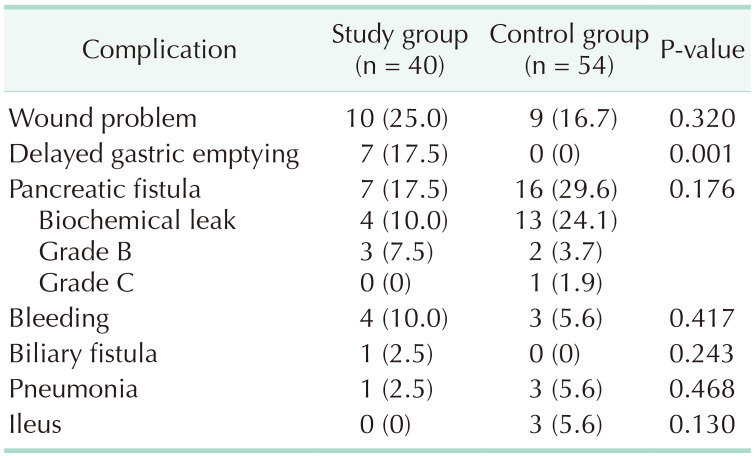
Twenty-five patients (62.5%) and 29 patients (53.7%) had at least 1 postoperative complication in the study and the control groups, respectively (Tables 1, 2). Grade A delayed gastric emptying occurred in 2 patients and grade B/C in 5, whereas there were no cases in the control group. Biochemical leak and grade B pancreatic fistula occurred in 4 and 3 patients, respectively; there were no grade C patients in the study group. In the control group, 13 patients had a biochemical leak and grade B and C fistula occurred in 2 and 1 patients, respectively. Only 3 patients in the control group had postoperative ileus. Seven patients in the study group had delayed gastric emptying, whereas there were no patients in the control group. There was no postoperative mortality in the study group, but 1 patient in the control group died 48 days after the operation due to sepsis.
Adverse effects of FCM were reported in 5 cases. Dyspepsia was reported in 2 cases and dizziness, hypertension, and pruritus were each reported in 1 case. The severity of dyspepsia and dizziness was moderate, and that of hypertension and pruritus was mild. All adverse effects were managed conservatively, or by medication, and all patients recovered well without any sequelae. There were no reports of headache, flushing, nausea, or vomiting.
The perioperative transfusion rate in the study group was 22.5% (9 of 40; 90% confidence interval [CI], 11.6–33.4); 6 patients (15.0%) during PD and 5 patients (12.5%) within 7 days after PD. Two patients required transfusion during both the intraoperative and postoperative periods.
The Hb level significantly increased from baseline to the day of the operation and also increased (but not significantly) between the day of the operation and the 4–6-week follow-up (Fig. 2). TSAT and ferritin levels also significantly increased on the day of the operation after administration of FCM but decreased at the 4–6-week follow-up. All of these parameters showed significantly higher levels at the follow-up compared to the baseline.
The perioperative transfusion rate in the study group was lower than that in the control group (22.5% vs. 38.9%; 90% CI, 11.6–33.4; 1-sided P = 0.046). The postoperative (within 7 days after PD) transfusion rate was also lower in the study group (12.5% vs. 29.6%; 1-sided P = 0.024) (Table 1). However, the EBL was significantly higher in the control group (P < 0.001). The patients in both groups were divided into 3 subgroups according to the EBL amount (Table 3). We compared transfusion rates using logistic regression after adjusting for the amount of EBL; there was no significant difference in the transfusion rate following the adjustment (odds ratio, 0.76; 95% CI, 0.27–2.15; 2-sided P = 0.607). Hb levels at the time of discharge (10.4 ± 1.4 mg/dL vs. 9.7 ± 1.2 mg/dL, P = 0.010), and at the 4–6-week follow-up (11.7 ± 1.3 mg/dL vs. 10.7 ± 1.3 mg/dL, P < 0.001), were significantly higher in the study group than in the control group.
We combined the control and the study groups, divided the transfusion group and the non-transfusion group, and compared them. The rate of FCM injection was 30.0% (9 of 30) in the transfusion group and 48.4% (31 of 64) in the non-transfusion group, but it was not significantly different (P = 0.092) (Table 4).
In this study, the perioperative transfusion rate of the study group was 22.5% (9 of 40), which was significantly lower than that in the control group (38.9%, 21 of 54; P = 0.046). However, due to greater blood loss in the control group (P < 0.001), there was no significant difference in transfusion rate between the 2 groups in a logistic regression model adjusted for blood loss (P = 0.607). In the study group, the Hb level was significantly higher on the day of the operation compared to the baseline (P < 0.001). Levels of Hb, TSAT, and ferritin were higher at the follow-up compared to baseline (P = 0.008, P = 0.033, and P < 0.001, respectively). Mild-to-moderate adverse effects of FCM were reported in 5 cases (20.0%), which were managed easily. To the best of our knowledge, this was the first study to investigate the efficacy of FCM in patients scheduled for PD.
In our previous study, a preoperative serum Hb level of <12 mg/dL was an independent predictor of perioperative transfusion, along with female sex, a long operation time, and portal vein resection [11]. In this study, 49.2% of PD patients (120 of 244) were anemic (Hb of <12 mg/dL), which in most cases was assumed to be due to IDA in association with a lack of iron intake, inability to absorb iron or chronic bleeding, etc. [11]. We hypothesized that, if the preoperative Hb in IDA patients scheduled for PD could be increased, the perioperative transfusion rate would decrease and long-term survival would improve. Intravenous iron, including FCM, has been used to rapidly improve hematological parameters including serum Hb, ferritin level, and TSAT, and to prevent unnecessary transfusion after gastrectomy [161718]. In a previous randomized, controlled trial performed at our institute, Kim et al. [18] reported that alternative anemia management strategies, such as oral iron supplementation and transfusion, were used more frequently in the observation group than in patients receiving FCM at 5–7 days after gastrectomy (6.9% vs. 1.4%; absolute difference, 5.5; 95% CI, 3.3–7.6; P = 0.006). In another randomized, controlled trial, transfusion was less frequent in the FCM group versus control group patients (0.97% vs. 5.1%; incidence rate ratio, 0.1; 95% CI, 0.01–0.85; P = 0.035) [17]. In that randomized, controlled trial, most of the operations were orthopedic, including knee and hip arthroplasties and spinal surgery; the rest were abdominal, gynecologic, urologic, plastic, and otolaryngologic surgeries.
This single-center, single-arm study assessed the efficacy and safety of FCM for patients scheduled for PD, in terms of the perioperative transfusion rate. FCM was infused 1–3 weeks before the operation in male patients with an Hb level of 7–13 g/dL, and in female patients with an Hb level of 7–12 g/dL. Compared to the historical control group, the transfusion rate significantly decreased from 38.9% to 22.5% in the study group (1-sided P = 0.046). However, there was no statistical difference in transfusion rate between the groups after adjusting for EBL (2-sided P = 0.607). The median EBL was significantly greater in the control group than the study group (600 mL [range, 300–3,800 mL] vs. 400 mL [range, 130–3,300 mL], P < 0.001), possibly because the operation time and surgeon experience differed between the groups; alternatively, the surgeons might have been more concerned about reducing bleeding in the study patients. We further investigated EBL in both groups: there was more proportion of patients with EBL of >1,000 mL in the control group than in the study group, and more proportion of patients in the study group with EBL of <500 mL (P = 0.005) (Table 3). In both groups, the transfusion rate increased with EBL (control group: 16%, 50%, and 66.7%; study group: 18.5%, 25%, 100%, in the subgroups with EBL of <500 mL, 500–1,000 mL, and >1,000 mL). After adjusting for EBL, the relative risk of transfusion in the study group was 0.46 (0.18–1.15, P = 0.095) in the univariate model and 0.76 (0.27–2.15, P = 0.607) in the multivariate model. Therefore, the reduced transfusion rate in the study group was mainly due to reduced blood loss compared to the control group; the effect of FCM administration was not statistically significant. We also performed subgroup analyses according to EBL, but the transfusion rate was not significantly different between the control and study groups (Table 3).
Levels of Hb, TSAT, and ferritin were significantly higher 4–6 weeks after PD than at the time of enrollment in the study group. Hb levels in the study group were also higher at the time of discharge, and at 4–6 weeks after PD, compared to the control group. The Hb level had improved after 12 weeks in patients who received FCM infusion, and levels of TSAT and ferritin were also higher, in a previous randomized trial [18]. In that trial, the Hb level showed a greater recovery in the FCM group than in the control group [17]. However, there have been no clinical trials regarding the effects of preoperative FCM, particularly in patients undergoing PD.
Randomized clinical trials reported that FCM was more effective in reversing IDA than an oral iron supplement [2224]. FCM also has fewer gastrointestinal adverse effects, such as nausea, vomiting, constipation, and diarrhea, than oral iron; the most common adverse effect is transient, asymptomatic hypophosphatemia [20]. Although one study reported several adverse effects of FCM, including fatigue, headache, dizziness, dyspepsia, and rash [24], no adverse events were reported in our study.
There was no delayed gastric emptying in the control group, but unexpectedly, it occurred in 7 patients in the study group. This was attributable to the fact that the study group applied more stringently in recording complications.
There were several limitations to the current study. First, it used a single-arm, single-center design; thus, although safety issues associated with FCM were relatively easy to manage, the effectiveness of FCM was more difficult to quantify. We compared the transfusion rate between study and historical control groups with similar Hb levels. Although the transfusion rate for the study group was significantly lower than that for the control group, the EBL differed between the groups. Hematologic parameters, including TSAT and ferritin levels, should have been measured before and after PD and compared in both groups. The median interval between FCM administration and the operation was 8 days (range, 7–33 days), which may be too short to observe a meaningful increase in Hb. However, as most of the patients were periampullary cancer patients, it was difficult to delay the operations. Moreover, the amount of FCM injected and the period between FMC administration and measurement of blood parameters were different. These factors have the potential to be a risk of increasing bias. The sample size of this study was small; future randomized, controlled, multicenter studies should be performed to confirm the efficacy and safety of FCM, as well as the transfusion and morbidity rates. To further clarify and elaborate the relationship between FCM administration and outcomes, univariate and multivariate analyses are required.
In conclusion, FCM for IDA patients scheduled for PD was associated with a lower perioperative RBC transfusion rate, likely due to an increase in the preoperative Hb level and reduced intraoperative blood loss. FCM can safely stabilize hematological parameters (Hb, ferritin, and TSAT) during the perioperative period in IDA patients scheduled for pancreaticoduodenectomy.
References
1. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007; 142:20–25. PMID: 17629996.

2. Wellner UF, Kulemann B, Lapshyn H, Hoeppner J, Sick O, Makowiec F, et al. Postpancreatectomy hemorrhage: incidence, treatment, and risk factors in over 1,000 pancreatic resections. J Gastrointest Surg. 2014; 18:464–475. PMID: 24448997.

3. Asari S, Matsumoto I, Toyama H, Yamaguchi M, Okada T, Shinzeki M, et al. Recommendation of treatment strategy for postpancreatectomy hemorrhage: Lessons from a single-center experience in 35 patients. Pancreatology. 2016; 16:454–463. PMID: 26935829.

4. Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000; 4:567–579. PMID: 11307091.

5. Park SJ, Kim SW, Jang JY, Lee KU, Park YH. Intraoperative transfusion: is it a real prognostic factor of periampullary cancer following pancreatoduodenectomy? World J Surg. 2002; 26:487–492. PMID: 11910485.

6. Yeh JJ, Gonen M, Tomlinson JS, Idrees K, Brennan MF, Fong Y. Effect of blood transfusion on outcome after pancreaticoduodenectomy for exocrine tumour of the pancreas. Br J Surg. 2007; 94:466–472. PMID: 17330243.

7. Hallet J, Mahar AL, Tsang ME, Lin Y, Callum J, Coburn NG, et al. The impact of peri-operative blood transfusions on post-pancreatectomy short-term outcomes: an analysis from the American College of Surgeons National Surgical Quality Improvement Program. HPB (Oxford). 2015; 17:975–982. PMID: 26301741.

8. Elwood NR, Martin AN, Turrentine FE, Jones RS, Zaydfudim VM. The negative effect of perioperative red blood cell transfusion on morbidity and mortality after major abdominal operations. Am J Surg. 2018; 216:487–491. PMID: 29475550.

9. Burrows L, Tartter P. Effect of blood transfusions on colonic malignancy recurrent rate. Lancet. 1982; 2:662.
10. Kneuertz PJ, Patel SH, Chu CK, Maithel SK, Sarmiento JM, Delman KA, et al. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011; 18:1327–1334. PMID: 21369744.

11. Park HM, Park SJ, Shim JR, Lee EC, Lee SD, Han SS, et al. Perioperative transfusion in pancreatoduodenectomy: the double-edged sword of pancreatic surgeons. Medicine (Baltimore). 2017; 96:e9019. PMID: 29245285.
12. Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984; 64:308–310. PMID: 6234037.

13. Waymack JP, Gallon L, Barcelli U, Trocki O, Alexander JW. Effect of blood transfusions on immune function. III. Alterations in macrophage arachidonic acid metabolism. Arch Surg. 1987; 122:56–60. PMID: 3492188.
14. Ghio M, Contini P, Mazzei C, Brenci S, Barberis G, Filaci G, et al. Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999; 93:1770–1777. PMID: 10029607.

15. Innerhofer P, Tilz G, Fuchs D, Luz G, Hobisch-Hagen P, Schobersberger W, et al. Immunologic changes after transfusion of autologous or allogeneic buffy coat-poor versus WBC-reduced blood transfusions in patients undergoing arthroplasty. II. Activation of T cells, macrophages, and cell-mediated lympholysis. Transfusion. 2000; 40:821–827. PMID: 10924610.

16. Yoon HM, Kim YW, Nam BH, Reim D, Eom BW, Park JY, et al. Intravenous iron supplementation may be superior to observation in acute isovolemic anemia after gastrectomy for cancer. World J Gastroenterol. 2014; 20:1852–1857. PMID: 24587663.
17. Khalafallah AA, Yan C, Al-Badri R, Robinson E, Kirkby BE, Ingram E, et al. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open-label, randomised controlled trial. Lancet Haematol. 2016; 3:e415–e425. PMID: 27570088.

18. Kim YW, Bae JM, Park YK, Yang HK, Yu W, Yook JH, et al. Effect of intravenous ferric carboxymaltose on hemoglobin response among patients with acute isovolemic anemia following gastrectomy: the FAIRY randomized clinical trial. JAMA. 2017; 317:2097–2104. PMID: 28535237.

19. Martínez-Escribano C, Arteaga Moreno F, Cuesta Peredo D, Blanco Gonzalez FJ, De la Cámara-de Las Heras JM, Tarazona Santabalbina FJ. Before-and-after study of the first four years of the enhanced recovery after surgery (ERAS®) programme in older adults undergoing elective colorectal cancer surgery. Int J Environ Res Public Health. 2022; 19:15299. PMID: 36430017.

20. Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs. 2015; 75:101–127. PMID: 25428711.

21. Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M. Anaemia Working Group España. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011; 107:477–478. PMID: 21841061.

22. Keeler BD, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, Acheson AG, et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017; 104:214–221. PMID: 28092401.
23. Reim D, Kim YW, Nam BH, Kim MJ, Yook JH, Park YK, et al. FAIRY: a randomized controlled patient-blind phase III study to compare the efficacy and safety of intravenous ferric carboxymaltose (Ferinject®) to placebo in patients with acute isovolemic anemia after gastrectomy. Study protocol for a randomized controlled trial. Trials. 2014; 15:111. PMID: 24708660.

24. Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009; 49:2719–2728. PMID: 19682342.

Fig. 2
Change of hematological parameters in the study group. (A) Hemoglobin (Hb) level change. Total Hb level significantly increased from baseline to operation day (P < 0.001) and also increased, but not significantly from operation day to follow-up 4 to 6 weeks later (P = 0.373). It finally increased from baseline to follow-up 4 to 6 weeks later (P = 0.008). Hb levels of patients with levels lesser than 10 maintained significantly higher in the follow-up period than in the baseline. Hb levels were compared using paired t-test. (B) Transferrin saturation (TSAT) level change. TSAT significantly increased on operation day after the administration of ferric carboxymaltose (FCM) (P < 0.001), but decreased 4 to 6 weeks later (P < 0.001). However, it maintained a significantly higher state at the follow-up period compared to the levels at baseline (P = 0.033). TSAT levels were compared using Wilcoxon signed-rank test. (C) Ferritin level change. Ferritin level also significantly increased at operation day after the administration of FCM (P < 0.001), but decreased 4 to 6 weeks later (P < 0.001). However, it maintained a significantly higher state at the follow-up period compared to the levels at baseline (P < 0.001). Ferritin levels were compared using Wilcoxon signed-rank test.
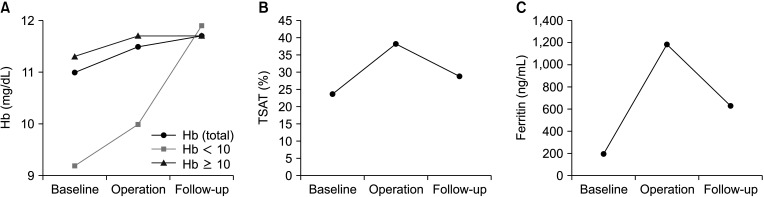
Table 1
Characteristics of the study and control group patients
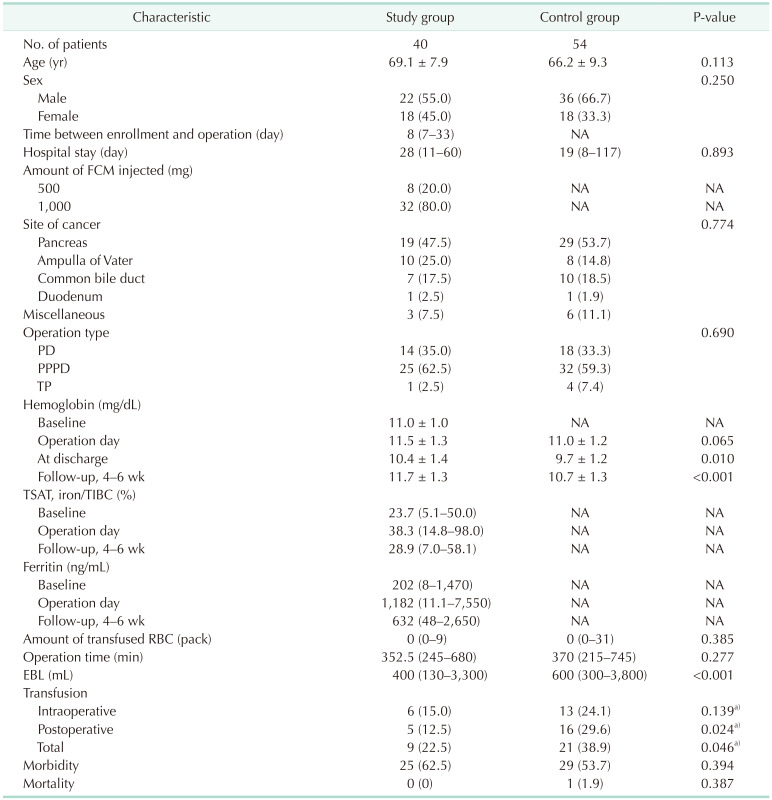
Values are presented as number only, mean ± standard deviation, or number (%).
NA, not applicapable; FCM, ferric carboxymaltose; PD, pancreatoduodenectomy; PPPD, pylorus-preserving pancreatoduodenectomy; TP, total pancreatectomy; TSAT, transferrin saturation; TIBC, total iron binding capacity; EBL, estimated blood loss.
a)One-sided P-value.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download