Abstract
Purpose
Primary hyperparathyroidism (PHPT) is caused by typical adenoma (TA), multiglandular disease (MD), or parathyroid carcinoma (PC), and in a smaller percentage of cases by atypical parathyroid tumor (APT). The objective of this study is the retrospective analysis of clinical features and parathyroid hormone (PTH)/calcium response to surgery in patients who underwent parathyroidectomy for symptomatic PHPT with histological evidence of APT.
Methods
We retrospectively reviewed our institutional experience in the management of PHPT from January 2016 to December 2021 focusing on those patients presenting APTs. We analyzed the clinical features of this disease and PTH/calcium response to surgical treatment in APTs compared to the other pathological conditions causing PHPT.
Results
In a cohort of 125 patients with PHPT we found 112 TAs (89.6%), 6 APTs (4.8%), 6 PCs (4.8%), and only 1 MD (0.8%). APTs in comparison to other parathyroid diseases showed peculiar features such as adhesion to the surrounding structures and a frequent intrathyroidal location, which may justify thyroid loboistmectomy adopted in most of the observed cases. APTs showed significantly higher preoperative PTH values compared to TA + MD and were relevant to PC.
Conclusion
Due to its rarity, there is a lack of specific indications in the management of APTs. Biochemical features observed in APT and PC can be related to similar biological behavior. However, some specific features observed preoperatively in some cases of PHPT might suggest presence of an APT, which could be helpful mostly in surgical and postoperative management. Further studies are required to confirm the results of the present preliminary report.
Primary hyperparathyroidism (PHPT) is one of the most frequent endocrine diseases, ranking third only after diabetes mellitus and thyroid disease [1], and it is the most common cause of hypercalcemia. PHPT shows a prevalence in female [2], and it is considered to have an indolent evolution in most cases, due to the slow tumoral growth; though there is a high variability of clinical and biochemical characteristics [3]. This pathology is characterized by inappropriate hypercalcemia or by serum parathyroid hormone (PTH) increase independent from calcium values [4]. PHPT can be suspected by incidental symptoms and signs of hypercalcemia, including bone fractures, nephrolithiasis, osteitis fibrosa cystica, osteoporosis, bone pain, myopathy, and neuropsychiatric impairment [5]. The latest 2022 World Health Organization (WHO) classification of parathyroid tumors [6] changed the term “parathyroid hyperplasia” applied to multiglandular parathyroid disease (MD) into multiglandular multiple parathyroid adenomas since affected glands are usually composed of multiple “clonal” neoplastic proliferations. The term “parathyroid hyperplasia” is now used primarily in the setting of secondary hyperparathyroidism related to chronic renal failure. Similarly, the new classification no longer endorses the use of the term “atypical parathyroid adenoma,” which is now replaced with the term “atypical parathyroid tumor” (APT) to reflect a parathyroid neoplasm of uncertain malignant potential. The most frequent cause of PHPT, as reported in the literature [5], is a solitary typical adenoma (TA) observed in 80%–85% of cases characterized by a single gland volume increase and normal gland’s cells replacement with neoplastic benign cells, not responding to any negative feedback from calcium values. MD accounts for 10%–15% of all cases of PHPT, while APT and parathyroid carcinoma (PC) rarely cause the disease, respectively, in 1.2% and 1.3% of cases [7]. Infrequently, PHPT is due to an ectopic adenoma with relevant clinical issues for correct localization and treatment [8]. APT is a lesion with suspicious clinical and histological features but which does not fit the WHO criteria for diagnosis of PC [6]. Due to its rarity, APT has been less studied compared to TA for the response to parathyroidectomy.
This retrospective observational cohort study was performed according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [9]. The study was conducted in accordance with the Declaration of Helsinki, but the protocol was not submitted to the evaluation of the Ethics Committee of the Umbria region or registered as a clinical trial due to the retrospective design of the research. All patients gave their informed consent to the use of their clinical data for research purposes at the time of surgery.
The present study was designed to analyze the clinical features and PTH/calcium response to surgery in patients who underwent parathyroidectomy for symptomatic PHPT with histological evidence of APT. We conducted a retrospective analysis of patients diagnosed with PHPT who were referred for surgical treatment to the Thoracic Surgery Unit, Department of Surgical Sciences, Santa Maria della Misericordia Hospital, University of Perugia Medical School. The study spanned a period of 6 years, from January 2016 to December 2021, and the analysis was conducted in accordance with the international guidelines for the management of PHPT [6]. Patients admitted with diagnosis of PHPT were identified in the department’s clinical records. Patients presenting with secondary and tertiary hyperparathyroidism were excluded. In all patients with PHPT, histology was revised by 2 blinded pathologists to confirm the correct diagnosis according to the criteria of the 2022 WHO classification of parathyroid tumors [6].
We identified 125 patients admitted to our institution with diagnosis of PHPT. All series included 56 males and 69 females with a mean age of 62.0 ± 12.8 years. According to the histological findings, the majority of patients (112, 89.6%) were classified as having TA (group A), 6 patients (4.8%) had APT (group B), 6 patients (4.8%) had PC (group C), and only 1 patient (0.8%) had MD (group D) (Table 1). Patients in group B included 4 females and 2 males with a mean age of 50.0 ± 14.4 years. Among all patients with PHPT, we focused on those with histological diagnoses of APT. General features, biochemical data, and surgical outcomes in the APT group were compared with those observed in the other groups. General management of all patients followed the Diagnostic-Therapeutic-Healthcare Protocol of the Italian National Society for Endocrine Surgery [10].
All patients before surgery underwent specific blood tests including intact PTH (iPTH), serum calcium and phosphorus, routine preoperative anesthetic assessment, sesta-methoxyisobutylisonitrile (sesta-MIBI), scintigraphy combined with ultrasound color-doppler imaging, and single photon emission computed tomography (SPECT)/CT scan in those cases with discordant results from the above investigations, in order to identify the pathological gland location. Selective parathyroidectomy or thyroid loboistmectomy was carried out as previously described by our group [1112]. Briefly focused parathyroidectomy with minimal skin incision under ultrasound guidance and limited dissection in the thyroid space for recurrent laryngeal nerve identification was carried out in cases of cervical solitary adenomas; wider unilateral exploration was required in MD. Loboistmectomy was carried out in cases of a missing identification of the pathological gland. In all the procedures, standard visualization of the inferior laryngeal nerve was carried out and intraoperative nerve monitoring with NIM 3.0 (Medtronic Italia S.p.A.) was also adopted in all cases. During all surgeries, we used intraoperative PTH (IO-PTH) assay. As per usual, 3 samples were collected, the first immediately before the anesthesia and the second and third ones respectively 10 and 20 minutes after removal of the pathologic gland. After surgery, iPTH on the postoperative day 1 and plasmatic calcium levels on the postoperative days 2 and 3 were dosed. In hypocalcemic patients, daily testing was performed until values were normalized. The type of procedure, surgical complications (recurrent laryngeal nerve lesion and cervical hematoma), surgical outcome (postoperative hypoparathyroidism and persistent PHPT), and histological features, including the size of the pathological gland, were analyzed. Hypoparathyroidism was defined as a concentration of iPTH under 10 ng/L (normal range, 10–65 ng/L) strictly correlated to hypocalcemia defined as plasmatic calcium under 1.0 mmol/L (normal range, 1.12–1.32 mmol/L). Treatment included, at the onset of muscular spasm and tetany associated with severe hypocalcemia, prompt intravenous calcium gluconate infusion and oral calcitriol (Rocaltrol, Roche SpS) 1.5 µg per day (0.5 µg every 8 hours) administration; and for asymptomatic patients, only oral calcium (Calcium-Sandoz Forte, Novartis Pharma SA) 3 g per day (1 g every 8 hours) plus calcitriol [13]. Persistent/recurrent PHPT was considered according to elevated iPTH value in the first days and during follow-up. Surgical follow-up was regularly carried out in the outpatient clinic for at least 12 months. A further, longer telephone follow-up was carried out for up to 42 months. For the statistical analysis, we studied the variables through descriptive statistics based on summary measures, and table distributions. A P-value of <0.05 was considered statistically significant. All of the data were analyzed using R statistical software ver. 4.2.3 (R Foundation for Statistical Computing).
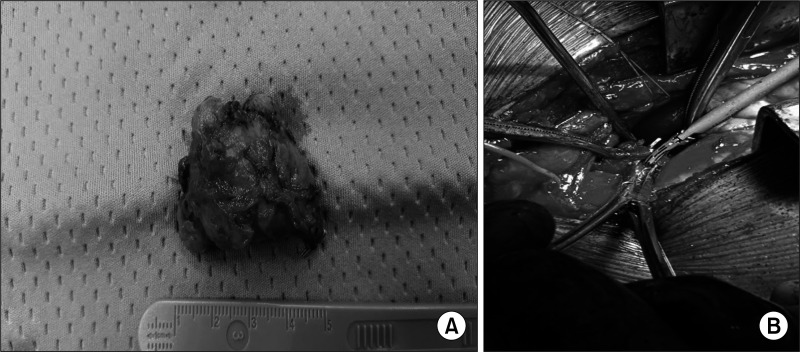
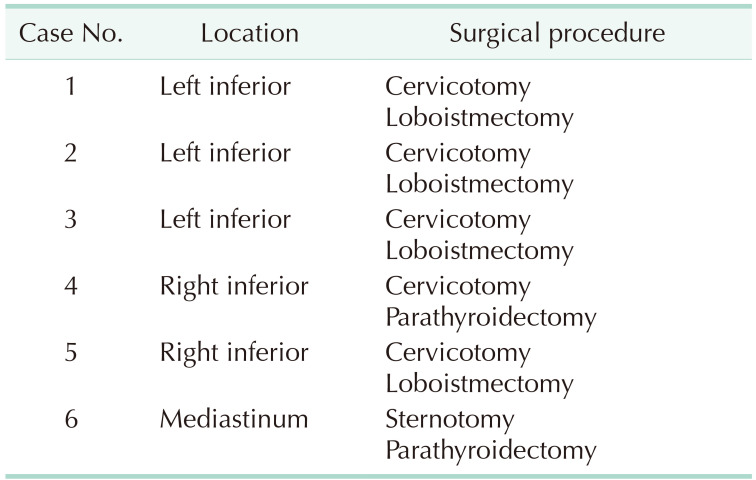
Out of 125 patients, 123 underwent a cervical approach, a selective parathyroidectomy, or, when required, a thyroid loboistmectomy if parathyroid missed identification occurred or due to intraoperative findings. Only 2 patients with mediastinal ectopic adenomas, required a sternotomy. The location of the pathological gland and the surgical procedure carried out in group B are summarized in Table 2. In 4 out of 6 patients, a thyroid loboistmectomy was carried out due to intrathyroidal location and to the strict adherence of the adenoma to the thyroid lobe. Among the other 2 patients with APT, 1 underwent inferior left selective parathyroidectomy through a cervicotomy and the other parathyroidectomy through a sternotomic access due to the ectopic mediastinal location of the adenoma, above the aortic arch (Fig. 1). After surgery, pathologic glands sizes resulted respectively 36.2 mm for APT, 30.33 mm for PC, 20.13 mm for TA, and 14.5 mm for MD.
We analyzed pre- and postoperative iPTH, IO-PTH, and serum calcium values in all patients presenting PHPT who underwent curative parathyroidectomy comparing group B with the other groups (Table 3). Mean preoperative iPTH in group B was significantly higher compared to groups A and D together (P = 0.002); although relevant but not significant compared to group C (P = 0.070). In group B, IO-PTH dosage observed 20 minutes after surgical excision showed a relevant drop compared to the preoperative values; though when considering the mean 20-minute IO-PTH, group B showed significantly (P = 0.008) higher values compared to group A + D. Still higher postoperative results, although not significant (P = 0.085), were observed when comparing APT with PC. PTH values resulted in normal range on the first postoperative day in all groups. Preoperative and postoperative calcium values in group B did not show significant differences compared to the other groups (Table 3). None of the patients, after surgery, showed inferior laryngeal nerve lesions, persistent hypoparathyroidism, or major postoperative complications. In group B, we observed a symptomatic hypocalcemia (6.7 mg/dL) on the 2nd postoperative day in only one patient, who was treated with calcium carbonate (3 g daily) for 10 days, till complete clinical and blood values resolved. None of the patients showed biochemical or clinical relapse of hyperparathyroidism during the follow-up.
Our findings confirm the extreme rarity of APT counting only 6 cases out of 125 operated PHPT over 6 years. In the APT group, intraoperative findings demonstrated macroscopic strict adhesion of the adenoma to the thyroid lobe and to the surrounding structures, and in 4 patients, the gland was located inside the thyroid lobe. In all the observed patients with APT, fibrous bands surrounding the pathological parathyroid were observed. For these reasons, in 4 out of 6 patients, a loboistmectomy with en bloc removal of the pathological gland was required to obtain radical excision. In our surgical series, higher pre- and postoperative PTH values, although not statistically significant, were noticed in APTs when compared to PCs. This observation confirms that APT is a lesion with suspicious clinical and histological features, though not completely fitting the full criteria of WHO 2022 for the diagnosis of PC. Furthermore, these findings justify indeed a more local “aggressive” behavior compared to ordinary adenomas. In all groups, PHPT was successfully treated with no residual or recurrent disease during follow-up.
The main bias and limitation of the present study is the limited number of APTs observed. This aspect cannot be overcome due to the rarity of this condition, despite a relevant number of total PHPTs observed. Our results can be considered as a preliminary observation with a need to be confirmed in larger studies. According to the literature [14], we can affirm that it is extremely difficult to intraoperatively distinguish an APT from a PC, and very often the morphological characteristics observed during surgery require a more radical and aggressive approach, limiting the adoption of a selective parathyroidectomy, as usually indicated in PHPT. In this scenario, it could be beneficial to know in advance if there is suspicion of APT in the preoperative setting, mostly considering the biochemical data which according to our observation and as similarly as reported by other authors [13], usually show higher preoperative iPTH and serum calcium levels compared to what is observed in PHPTs caused by other parathyroid pathological conditions. Furthermore, IO-PTH in patients with APT was also found to be significantly higher after surgery compared to group A + D, and relevant higher compared to group C. This aspect, if confirmed in larger series, might be considered as a key of interpretation of the high PTH and IO-PTH levels observed before and during surgery and as criteria of suspicion not only for PCs but also for APT. Regarding iPTH response to parathyroidectomy, no difference was observed in APTs compared to the other groups. Once again, our study confirms the pivotal role of the preoperative study for PHPT, in order to precisely localize the adenoma allowing consequently a correct preoperative surgical plan and avoiding a wrong or a missed identification of the pathologic gland. This approach is recommended together with IO-PTH assay to contain the risk of residual or recurrent PHPT. Sesta-MIBI scintigraphy and SPECT/CT, at the moment, are the most used and frequent methods to study parathyroid glands disease [715]. In our study, all patients underwent sesta-MIBI scintigraphy plus ultrasound colordoppler imaging, which precisely provided the pathological gland location in 89 out of 125 as a single method. The other cases required combined parathyroid imaging techniques including SPECT/CT for a concordant localization. All patients with APT required multimodality imaging, probably due to the intrathyroidal and the ectopic mediastinal location that we observed. The pathological examination must provide a clear picture of the histological findings to properly classify the rare condition of APT. Although a demanding diagnosis, the 2022 WHO classification of parathyroid tumors, previously cited, precisely defined the criteria to refer a parathyroid adenoma to the category of APT. According to these criteria as confirmed by other authors [16], the presence of fibrosis and a thick capsule are specific gross characteristics of these tumors. Histology usually describes different growth patterns, nuclear atypia, and increased mitotic activity (Fig. 2). The presence of necrotic spots, vascular and perineal invasion can be detected by CD31 analysis. Immunohistochemistry for Ki-67, galectin-3, GATA3, and B-cell lymphoma 2 can be used for the diagnosis when positive staining is detected during the analysis [1718].
APT can be defined as a rare parathyroid neoplasm that expresses features similar to what is observed in PC, usually associated with symptomatic hyperparathyroidism with typically high levels of preoperative iPTH [7141920]. The principal perspective in this clinical setting is to define features of suspicion between APT and PC, based on preoperative and postoperative criteria in order to define an appropriate surgical approach and management to this rare condition.
In conclusion, we can affirm that APT presents specific features compared mostly to TA than to PC, particularly adhesion to the surrounding structures and intrathyroidal location, which may justify the indication of thyroid loboistmectomy in some cases. In our observation, APTs were characterized by significantly higher preoperative iPTH and IO-PTH values compared to TA and MD together, and higher values, although not significant compared to PC. These findings confirm similar biochemical features of APTs and PCs and, simultaneously, indicate a similar biological attitude. The analysis via larger series is required to better define the features of this rare condition and to assess specific recommendations of management.
References
1. Vaira V, Verdelli C, Forno I, Corbetta S. MicroRNAs in parathyroid physiopathology. Mol Cell Endocrinol. 2017; 456:9–15. PMID: 27816765.

2. Saponaro F, Cetani F, Repaci A, Pagotto U, Cipriani C, Pepe J, et al. Clinical presentation and management of patients with primary hyperparathyroidism in Italy. J Endocrinol Invest. 2018; 41:1339–1348. PMID: 29616419.

3. Aldinger KA, Hickey RC, Ibanez ML, Samaan NA. Parathyroid carcinoma: a clinical study of seven cases of functioning and two cases of nonfunct ioning parathyroid cancer. Cancer. 1982; 49:388–397. PMID: 7053835.

4. Bilezikian JP, Bandeira L, Khan A, Cusano NE. Hyperparathyroidism. Lancet. 2018; 391:168–178. PMID: 28923463.

5. Gasser RW. Clinical aspects of primary hyperparathyroidism: clinical manifestations, diagnosis, and therapy. Wien Med Wochenschr. 2013; 163:397–402. PMID: 23990260.

6. Erickson LA, Mete O, Juhlin CC, Perren A, Gill AJ. Overview of the 2022 WHO classification of parathyroid tumors. Endocr Pathol. 2022; 33:64–89. PMID: 35175514.

7. Galani A, Morandi R, Dimko M, Molfino S, Baronchelli C, Lai S, et al. Atypical parathyroid adenoma: clinical and anatomical pathologic features. World J Surg Oncol. 2021; 19:19. PMID: 33472651.

8. DeLellis RA. Parathyroid tumors and related disorders. Mod Pathol. 2011; 24 Suppl 2:S78–S93. PMID: 21455204.

9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014; 12:1495–1499. PMID: 25046131.

10. Rosato L, Raffaelli M, Bellantone R, Pontecorvi A, Avenia N, Boniardi M, et al. Diagnostic, therapeutic and healthcare management protocols in parathyroid surgery: II Consensus Conference of the Italian Association of Endocrine Surgery Units (U.E.C. CLUB). J Endocrinol Invest. 2014; 37:149–165. PMID: 24497214.

11. Polistena A, Lucchini R, Monacelli M, Triola R, Avenia S, Barillaro I, et al. Current indications for surgical treatment of primary hyperparathyroidism in the elderly. Am Surg. 2017; 83:296–302. PMID: 28316315.

12. Polistena A, Sanguinetti A, Lucchini R, Galasse S, Avenia S, Monacelli M, et al. Surgical treatment of secondary hyperparathyroidism in elderly patients: an institutional experience. Aging Clin Exp Res. 2017; 29(Suppl 1):23–28. PMID: 27830521.

13. Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016; 151:959–968. PMID: 27532368.

14. Cetani F, Marcocci C, Torregrossa L, Pardi E. Atypical parathyroid adenomas: challenging lesions in the differential diagnosis of endocrine tumors. Endocr Relat Cancer. 2019; 26:R441–R464. PMID: 31085770.

15. Martínez-Rodríguez I, Martínez-Amador N, de Arcocha-Torres M, Quirce R, Ortega-Nava F, Ibáñez-Bravo S, et al. Comparison of 99mTc-sestamibi and 11C-methionine PET/CT in the localization of parathyroid adenomas in primary hyperparathyroidism. Rev Esp Med Nucl Imagen Mol. 2014; 33:93–98. PMID: 24125595.

16. Kong SH, Kim JH, Kim SW, Shin CS. Radioactive parathyroid adenomas on sestamibi scans: low parathyroid hormone secretory potential and large volume. Endocrinol Metab (Seoul). 2021; 36:351–358. PMID: 33820395.

17. Weber T, Dotzenrath C, Dralle H, Niederle B, Riss P, Holzer K, et al. Management of primary and renal hyperparathyroidism: guidelines from the German Association of Endocrine Surgeons (CAEK). Langenbecks Arch Surg. 2021; 406:571–585. PMID: 33880642.

18. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017; 67:93–99. PMID: 28094848.

19. Clark CM, Payne SJ, Warrick JI, Goldenberg D. Atypical parathyroid adenoma with diffuse fibrosis. Ear Nose Throat J. 2017; 96:57–58. PMID: 28231363.

20. Stojadinovic A, Hoos A, Nissan A, Dudas ME, Cordon-Cardo C, Shaha AR, et al. Parathyroid neoplasms: clinical, histopathological, and tissue microarray-based molecular analysis. Hum Pathol. 2003; 34:54–64. PMID: 12605367.

Fig. 2
Histopathological findings. (A) Wide view of well-circumscribed multinodular neoplasia stained with H&E. The tumor shows numerous broad fibrous bands indicated by black arrowheads. (B) The tumor consists of both intermediate-sized chief cells (black asterisks) and oxyphilic cells (white arrowheads), growing either in a follicular or solid pattern. (C) Immunohistochemical staining showing nuclear GATA-3 positivity, confirming the parathyroid origin of the tumor cells. (D) Immunohistochemical staining showing membrane Bcl-2 positivity, further supporting the parathyroid origin of the tumor cells. (E) Perineural invasion indicated by S-100 immunostaining of nervous structures. (F) Vascular invasion shown by CD31 immunostaining. The tumor lacks consistent mitotic activity, necrosis, and clear evidence of invasive growth through the capsule into adjacent structures, supporting the diagnosis of atypical parathyroid adenoma. Original magnifications: A, ×16; B–D and F, ×200; E, ×100.
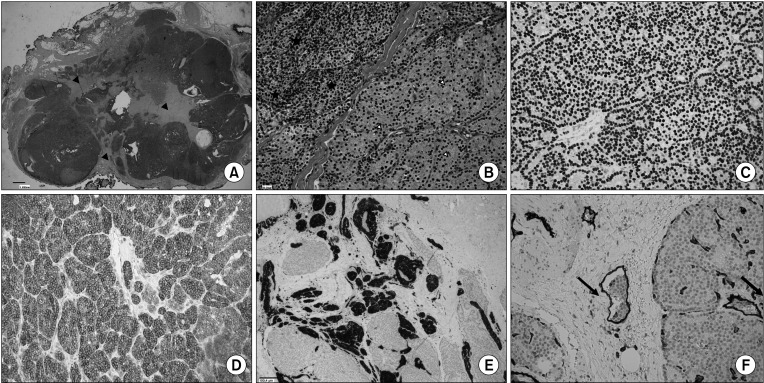
Table 3
PTH/calcium response to surgical treatment in the different groups
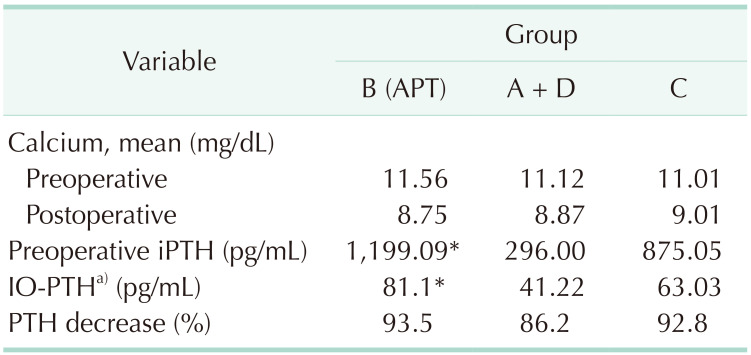
PTH, parathyroid hormone; APT, atypical parathyroid tumor; iPTH, intact PTH; IO-PTH, intraoperative PTH.
Please refer to Table 1 for the classification of groups.
a)Twenty minutes after parathyroidectomy.
*P < 0.05 compared to groups A + D.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download