Abstract
Purpose
Deceased donor liver transplantation (DDLT) recipients in Korea are generally sicker due to an increasing organ shortage. In the present study, the risk factors for early 30-day liver graft failure after DDLT were identified.
Methods
From August 2017 to February 2021, 265 adult DDLTs were performed. The characteristics of patients with and without 30-day graft failure were compared.
Results
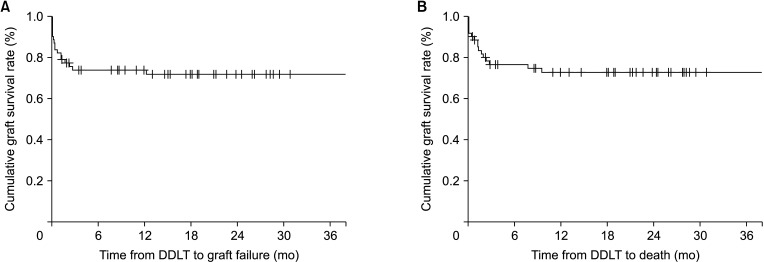
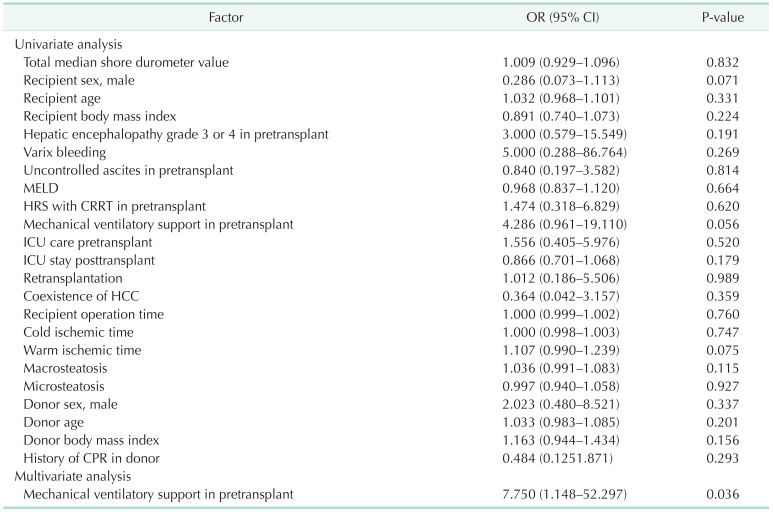
Liver graft failure occurred in 11 patients (17.7%) after DDLT. Baseline and perioperative characteristics of donors and recipients were not statistically significantly different between the 2 groups. The cumulative graft and overall survival rates at 6 months were 83.9% and 88.7%, respectively. Multivariate analysis showed ventilator support in the pretransplant period was a predisposing factor for 30-day graft failure after DDLT.
Liver transplantation (LT) is a life-saving procedure for patients with a wide range of end-stage liver diseases (ESLDs). Liver disease is progressive in nature and patient care is complex and challenging. Consequently, transplant candidates may require admission to the intensive care unit (ICU) while awaiting transplantation [1]. Significant improvements in surgical technique, medical management, and advances in immunosuppression therapy have contributed to the success of LT. In recent studies, the overall outcome of LT was shown to depend on a combination of factors including recipient condition, donor organ quality, and transplant center volume [23].
Despite multiple efforts, an agreed-upon definition of “too sick to transplant” or standardized guidelines for when a critically ill patient should be removed from a transplant waiting list currently do not exist [4]. Criteria used to delist a sick patient are transplant program-dependent. Ventilatory support and vasopressor therapy in a cirrhotic patient are considered contraindications to proceeding with transplantation [1]. In the current organ allocation policy based on urgency, patients at the highest risk of mortality are offered deceased donor LT (DDLT) first [5]. Currently, DDLT recipients in Korea are generally sicker due to increasing organ shortage [6]. Accordingly, from 2007 to 2016, LT patients were older and sicker [7].
Futile LT refers to a situation where the procedure is unlikely to be successful, either because the patient is too sick or because the liver is too damaged. In the present study, the risk factor for early liver graft failure after DDLT were identified based on the assessment of liver graft hardness using a shore durometer.
From August 2017 to February 2021, 265 adult DDLTs were performed in Samsung Medical Center. The Institutional Review Board (IRB) of Samsung Medical Center approved the present study (No. SMC-023-05-059). The IRB waived the need for patient consent because this was a retrospective observational study that used data from patient medical records. The study complied with the Declaration of Helsinki.
Patients were excluded based on the following criteria: living donor LT, retransplantation, pediatric LT (<18 years of age), synchronous multiple organ transplants, receiving a split graft, liver graft use after donor cardiac death, incomplete medical records, or follow-up loss.
The following donor characteristics were collected: sex, age, body mass index (BMI), length of ICU stay, steatosis of liver graft, and cause of brain death. Recipient data included sex, age, BMI, etiology for LT, Model for End-stage Liver Disease (MELD) score, cold ischemic time, warm ischemic time, length of follow-up, and graft and overall survival time.
The surgeon who performed the surgery subjectively evaluated the liver hardness by palpation during the operation before liver harvest. Liver wedge resection was routinely performed to detect fatty liver graft. Hepatic hardness was measured using a handheld shore durometer (Shore Hardness Tester HT6510OO, Guangzhou Landtek Instruments) with values ranging from 0 to 100 shore units with higher values indicating harder tissue. In the operation field, a thin transparent film was applied on the liver surface and the shore durometer was pressed perpendicularly on the liver surface. The number on the instrument panel was determined as hepatic hardness, and a mean value was obtained from 12 measurements at different sites.
Graft failure within 30 days was defined as lack of function of the implanted liver that resulted in retransplantation or death. National criteria for retransplantation establish primary nonfunction if 2 of the following criteria are present: AST, >10,000 IU/L; international normalized ratio, >3.0; arterial lactate, >3 mmol/L; or absence of bile production [8]. Graft failure was defined as requiring retransplantation. Postoperative mortality was recorded with cause of death. Graft survival was calculated from DDLT to graft failure and overall survival was calculated from DDLT to death.
Data management and analyses were performed with the IBM SPSS Statistics ver. 22 (IBM Corp.). Categorical data are presented as proportions with stratification based on 30-day graft failure. Bivariate associations of baseline factors with and without 30-day graft failure were examined using the chi-square or Fisher exact tests. Continuous data are presented as median and range based on 30-day graft failure. Continuous variables of patients with and without 30-day graft failure were compared using the Mann-Whitney U-test. Multivariate binary regression was performed using a variable with a P-value of <0.1 in univariate analysis. A P-value of <0.05 was considered statistically significant.
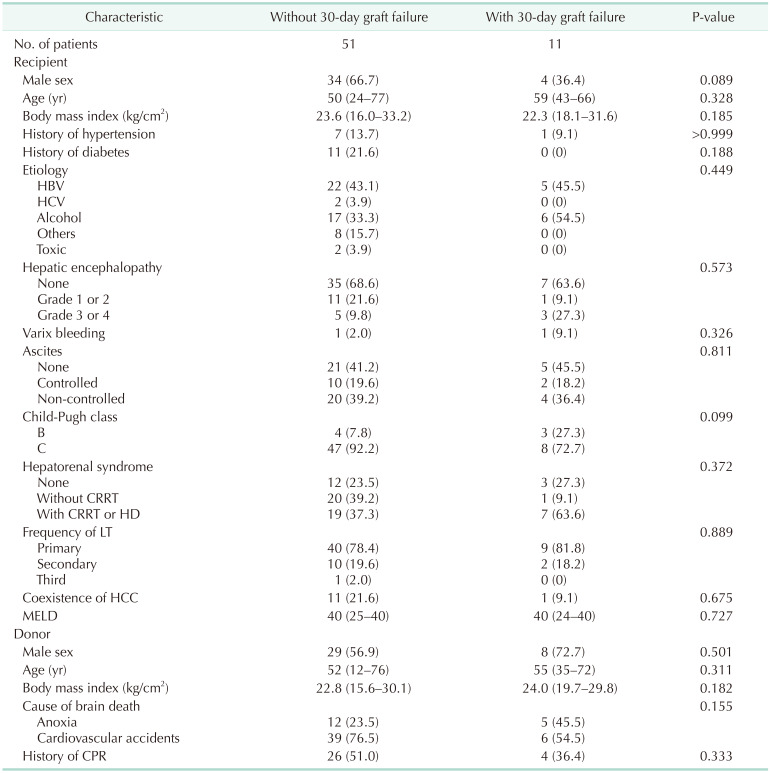
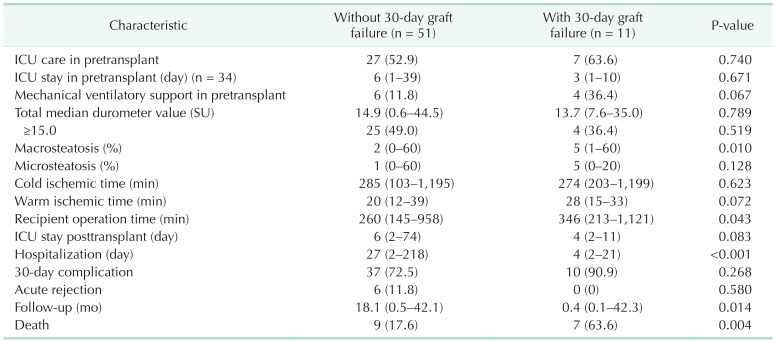
Liver graft failure after DDLT occurred in 11 patients (17.7%). In recipient baseline characteristics, sex, age, BMI, history of hypertension or diabetes, etiology, hepatic encephalopathy, varix bleeding, ascites, Child-Pugh class, hepatorenal syndrome, retransplantation, coexistence of hepatocellular carcinoma, and MELD score did not differ between patients with or without 30-day graft failure (Table 1). In donor baseline characteristics, statistically significant differences were not found in sex, age, BMI, cause of brain death, and history of cardiopulmonary resuscitation between the 2 groups (patients with 30-day graft failure and patients without 30-day graft failure).
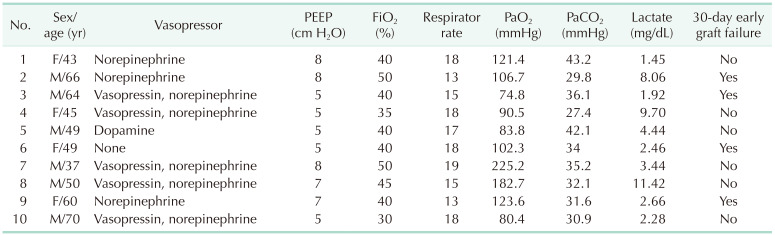
More than 50% of the patients (n = 34) were managed in the ICU before DDLT. In the 30-day graft failure group, 4 patients (36.4%) required mechanical ventilatory support. Inotropes, ventilator information, and arterial blood gas analysis of patients with mechanical ventilatory support during the pretransplant period are summarized in Table 2. Median macrosteatosis and median recipient operation time in the 30-day graft failure group were significantly longer than in the without 30-day graft failure group. Thirty-four patients were managed in the pretransplant ICU. Median pretransplant ICU stay was 6 days (range, 1–39 days) in the without 30-day graft failure group (n = 27) and 3 days (range, 1–10 days) in the with 30-day graft failure group (n = 7). Median hospitalization and follow-up duration in the patients with graft failure were shorter than in the patients without graft failure because graft failure subjects experienced early death (Table 3). In the 30-day graft failure group, 7 patients (63.6%) died and in the without graft failure group, 9 patients (17.6%) died. In the 30-day graft failure group, 4 patients underwent retransplantation and lived. Median shore durometer value did not differ between the 2 groups (Fig. 1).
Due to the complexity of surgical intervention and critical status of LT patients, a large proportion of the overall complications occur within the first postoperative week. Primary nonfunction or early graft failure is diagnosed within 10, 14, or 30 days from the primary DDLT, in which liver failure leading to death or the need for retransplantation occurs [9]. In the present study, early graft failure was defined as the lack of function of the implanted liver within 30 days after implantation that resulted in retransplantation or death [8]. Thus, in the present study, graft failure within 30 days after DDLT was investigated. The incidence rate of 30-day graft failure after DDLT was 17.7%. The need for mechanical ventilatory support in the pretransplant period was strongly associated with increased risk of early graft failure and death after DDLT in the present study. Shore durometer value for assessing liver hardness during liver graft harvest in the deceased donor did not influence outcomes after DDLT.
Several donor and recipient factors can influence the outcomes of DDLT such as donor age, cause of donor death, steatosis in liver graft, ischemic time, severity of liver disease, presence of coexisting medical disease, and waiting time on the transplant list. These factors play a vital role in determining the success rate, graft survival, and patient recovery. Intraoperative anesthetic management and surgical techniques can also strongly influence postoperative patient and graft function. All these factors can significantly affect graft functional restoration and patient outcome [10].
In the present study, mechanical ventilatory support in the pretransplant period was closely associated with early graft failure in DDLT patients. Previously, preoperative mechanical intubation was identified as an independent risk factor for decreased patient and graft survival [11]. Advanced liver disease frequently mandates ICU admission. The admission to ICU is associated with high mortality and LT becomes the only definitive therapeutic option for a decompensated cirrhotic patient. To date, one of the most complex decisions transplant surgeons face is when an extremely ill candidate is no longer suitable for LT. In a previous study, ICU patients after DDLT reportedly had a lower rate of survival compared with non-ICU patients after DDLT [12]. Indications for mechanical ventilatory support in patients with ESLD include acute respiratory failure due to hepatic encephalopathy, acute respiratory distress syndrome secondary to sepsis or pneumonia, pulmonary edema related to portal hypertension, coexisting liver disease, increased labored breathing due to ascites or hepatopulmonary syndrome, and inability to protect the airway due to altered mental state [13]. In the ICU, deteriorating patients with ESLD awaiting LT develop multiple organ system failure requiring mechanical ventilatory support, intermittent or continuous renal replacement therapy, and pharmacologic hemodynamic support. Vasopressor requirement and intubation have been considered contraindications to transplantation and regarded as criteria for delisting [1].
Potential outcomes after DDLT in patients who received mechanical ventilatory support in the pretransplant period included increased risk of complications associated with respiratory issues posttransplant, longer hospital stays and higher healthcare costs, higher rates of mortality and morbidity following transplantation, greater likelihood of infection and organ rejection, and need for ongoing respiratory support and management posttransplantation [11012131415].
In a previous study, hepatic hardness measured using a shore durometer reportedly correlated with the degree of liver fibrosis [16]. High value in shore durometer measurement was a more effective parameter for predicting posthepatectomy liver failure compared with other indicators evaluated before hepatectomy [16]. Thus, in the present study, the outcomes of DDLT were evaluated based on liver hardness assessed using a shore durometer; however, the shore durometer values did not reflect outcomes after DDLT. In a previous study, perceived low quality of donor livers was reportedly not associated with worse graft or recipient outcomes during the first week or first year after LT compared with standard quality donors [2]. These results are consistent with reports from Western countries that showed comparable short- and long-term survival outcomes between rescue allocation and standard allocation liver donors [17]. Acceptance of a liver donor depends primarily on the perception of the donor's liver quality as assessed by the transplant team combined with several known risk factors, such as degree of steatosis and cold ischemia time. These comparable results may have been achieved due to careful donor and recipient matching and are in agreement with the traditional and widely accepted approach of balancing risk that proposes lower risk recipients will tolerate a poor-quality liver graft better and sicker patients would benefit more from receiving a good-quality donor liver [18]. In the present study, perceived low-quality donor livers were more frequently allocated to less severely ill recipients.
The present study had several limitations. First, due to the retrospective design, inherent limitations existed. Second, detailed information such as cause of mechanical ventilatory support, duration of mechanical ventilation, and inotrope dose was unknown. Third, the number of cases was small; thus, definitive conclusions cannot be drawn.
In conclusion, present study indicates that cautious decision is required when allocating DDLT in critically ill patients on mechanical ventilatory support. A much larger group of ICU patients, likely from a multicenter study, is needed to better define criteria for a successful LT in ESLD patients on mechanical ventilatory support.
References
1. Findlay JY, Fix OK, Paugam-Burtz C, Liu L, Sood P, Tomlanovich SJ, et al. Critical care of the end-stage liver disease patient awaiting liver transplantation. Liver Transpl. 2011; 17:496–510. PMID: 21506240.

2. Dirchwolf M, Becchetti C, Stampf S, Haldimann C, Immer F, Beyeler F, et al. The impact of perceived donor liver quality on post-transplant outcome. ANZ J Surg. 2023; 93:918–925. PMID: 36708059.

3. Chung YK, Park CS, Kang SH. Association between institutional liver transplantation cases volume and mortality: a meta-analysis of Korea-nationwide cohort studies using Korean National Healthcare Insurance Service database. Ann Liver Transplant. 2022; 2:8–14.

4. Flores A, Asrani SK. The donor risk index: A decade of experience. Liver Transpl. 2017; 23:1216–1225. PMID: 28590542.

5. Kim MS. Modification of emergency status in deceased donor liver allocation: evidence for Korean model of end-stage liver disease (MELD) system. J Korean Soc Transplant. 2016; 30:51–58.

6. Ha HS, Hong JJ, Kim IO, Lee SR, Lee AY, Ha TY, et al. Deceased donor liver transplantation under the Korean model for end-stage liver disease score-based liver allocation system: 2-year allocation results at a high-volume transplantation center. Korean J Transplant. 2019; 33:112–117. PMID: 35769978.

7. Gil E, Kim JM, Jeon K, Park H, Kang D, Cho J, et al. Recipient age and mortality after liver transplantation: a population-based cohort study. Transplantation. 2018; 102:2025–2032. PMID: 30153223.

8. Diaz-Nieto R, Lykoudis P, Robertson F, Sharma D, Moore K, Malago M, et al. A simple scoring model for predicting early graft failure and postoperative mortality after liver transplantation. Ann Hepatol. 2019; 18:902–912. PMID: 31405576.

9. Masior Ł, Grąt M. Primary nonfunction and early allograft dysfunction after liver transplantation. Dig Dis. 2022; 40:766–776. PMID: 35114676.

10. Bolondi G, Mocchegiani F, Montalti R, Nicolini D, Vivarelli M, De Pietri L. Predictive factors of short term outcome after liver transplantation: a review. World J Gastroenterol. 2016; 22:5936–5949. PMID: 27468188.

11. Markmann JF, Markmann JW, Markmann DA, Bacquerizo A, Singer J, Holt CD, et al. Preoperative factors associated with outcome and their impact on resource use in 1148 consecutive primary liver transplants. Transplantation. 2001; 72:1113–1122. PMID: 11579310.

12. Asrani SK, Saracino G, O'Leary JG, Gonzalez S, Kim PT, McKenna GJ, et al. Recipient characteristics and morbidity and mortality after liver transplantation. J Hepatol. 2018; 69:43–50. PMID: 29454069.

13. Huang CT, Lin HC, Chang SC, Lee WC. Pre-operative risk factors predict post-operative respiratory failure after liver transplantation. PLoS One. 2011; 6:e22689. PMID: 21829646.

14. Smetana GW, Lawrence VA, Cornell JE. American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006; 144:581–595. PMID: 16618956.

15. Neviere R, Edme JL, Montaigne D, Boleslawski E, Pruvot FR, Dharancy S. Prognostic implications of preoperative aerobic capacity and exercise oscillatory ventilation after liver transplantation. Am J Transplant. 2014; 14:88–95. PMID: 24354872.

16. Yoon YC, Lee JS, Park SU, Kwon JH, Hong TH, Kim DG. Quantitative assessment of liver fibrosis using shore durometer. Ann Surg Treat Res. 2017; 93:300–304. PMID: 29250508.

17. Marcon F, Schlegel A, Bartlett DC, Kalisvaart M, Bishop D, Mergental H, et al. Utilization of declined liver grafts yields comparable transplant outcomes and previous decline should not be a deterrent to graft use. Transplantation. 2018; 102:e211–e218. PMID: 29702538.

18. Badawy A, Kaido T, Uemoto S. Current status of liver transplantation using marginal grafts. J Invest Surg. 2020; 33:553–564. PMID: 30457408.

Fig. 1
Median shore durometer values in patients with and without 30-day graft failure. SU, shore unit.
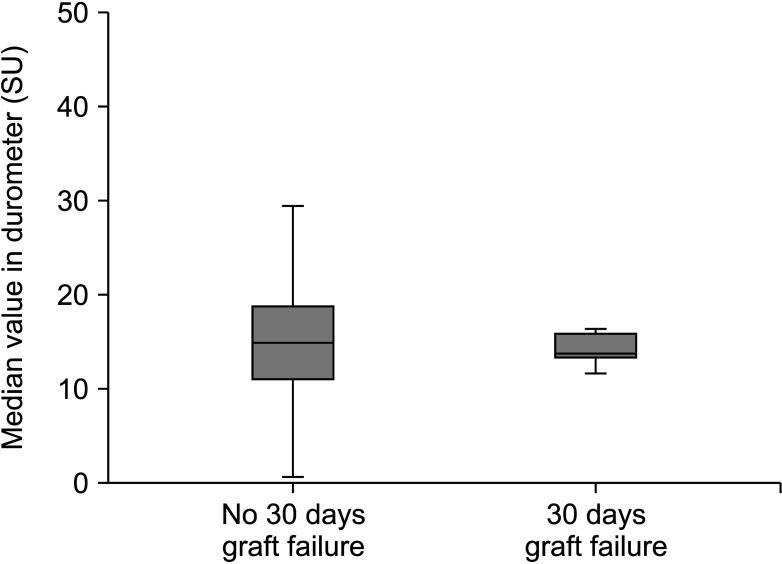
Fig. 2
Cumulative survival analysis of deceased donor liver transplantation (DDLT). (A) Graft survival and (B) overall survival.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download