Abstract
Purpose
We investigated the clinical characteristics and treatment outcomes of symptomatic Meckel diverticulum (MD) in adolescents by comparison with children and adults.
Methods
We retrospectively reviewed the medical records of patients who underwent symptomatic MD surgery from January 2002 to December 2019. Demographic information, clinical presentations, preoperative evaluations, operative variables, postoperative outcomes, and pathologic findings were collected. We performed analyses by dividing all patients into three groups according to age at surgery: child group (<10 years), adolescent group (10–19 years), and adult group (≥20 years).
Results
Forty-three patients underwent symptomatic MD surgery (the child group, 14; the adolescent group, 17; and the adult group, 12). Vomiting and intestinal obstruction decreased significantly with age (P = 0.042 and 0.001), whereas hematochezia and gastrointestinal bleeding showed an increasing trend with age, although not statistically significant (P = 0.064 and 0.064). Ultrasound performance decreased significantly with age (P = 0.002), whereas CT performance showed an increasing trend with age, although not statistically significant (P = 0.193). Preoperative diagnosis rate increased significantly with age (P = 0.029). Laparoscopic surgery was performed significantly more in the adult group than in other groups (P = 0.001). The sizes of MD were significantly greater in the adolescent group than in other groups (P = 0.006 and 0.002).
Conclusion
The clinical characteristics and treatment outcomes of symptomatic MD in adolescents exhibit a transitional pattern between children and adults. Therefore, it is important for clinicians to recognize that adolescent patients with symptomatic MD have the characteristics of both children and adult patients to ensure optimal care.
Meckel diverticulum (MD) is the most common congenital anomaly of the gastrointestinal (GI) tract that affects approximately 1%–2% of the population [123]. The first recorded description of MD is attributed to Wilhelm Fabricius Hildanus in 1598 [14], and then, it was described in detail by Johann Friedrich Meckel in 1809 [5]. Meckel showed that incomplete obliteration of the vitelline duct during the fifth gestational week results in intestinal blind pouches (MD), enterocysts, intestinal-umbilical fistulas, and mesodiverticular bands [6]. A patient with MD usually remains asymptomatic throughout the lifetime, and the lifetime incidence rate of complications is approximately 4.2%-9% [378]. Symptomatic MD is presented by diverticulitis, intestinal obstruction, and GI bleeding. Several previous studies have reported that the clinical presentation of symptomatic MD differs between pediatric patients and adult patients; however, the conclusions drawn by these studies are not consistent [3691011]. According to Park et al. [6], intestinal obstruction is the most common presentation in pediatric patients (age of <11 years) and GI tract bleeding in adult patients. Contrarily, Chen et al. [9] reported that GI tract bleeding and intestinal obstruction were the most common presentations in pediatric patients (age of <18 years), and diverticulitis in adult patients. Previous studies have reported differing conclusions on the most common presentations of symptomatic MD in different age groups. One major factor contributing to this variability is the lack of consensus on the age cutoffs for pediatric patients. Notably, the inclusion or exclusion of adolescent patients (age of 10–19 years) in the pediatric or adult patient groups has been a subject of debate and may significantly affect the study results. Therefore, in this study, we aimed to compare the clinical characteristics and treatment outcomes of symptomatic MD among pediatric, adolescent, and adult patients and specifically investigate the differences in presentation and management of symptomatic MD across different age groups, with a particular focus on adolescent patients.
This study was approved by the Institutional Review Board of Ulsan University Hospital (No. UUH 2020-11-018). It was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
We retrospectively reviewed the medical records of patients who underwent MD surgery from January 2002 to December 2019. Only patients who underwent symptomatic MD surgery were included in the study. A symptomatic MD was defined as MD that considered the cause of clinical symptoms based on both surgical findings and pathologic findings. Asymptomatic patients for whom MD was incidentally detected during the operation were excluded from the study.
Demographic information, clinical presentation (symptoms and complications), preoperative evaluation, operative variables, postoperative outcomes, and pathologic findings were retrieved from patients’ medical records. Symptoms were recorded using a standardized form consisting of historical and physical examination variables measured in our hospital, categorized into abdominal pain, vomiting, abdominal distension, tenderness, fever, and hematochezia. Complications were categorized into diverticulitis with or without perforation, intestinal obstruction with or without intussusception, and GI tract bleeding. Preoperative evaluation included laboratory tests (hemoglobin, WBC count, CRP concentration) on the day of hospital admission and advanced imaging diagnostic tests such as CT, ultrasonography (USG), upper and lower GI endoscopy, capsule endoscopy, and Meckel scan. Operative variables included surgical procedure (diverticulectomy, wedge resection, small bowel resection, and anastomosis), surgical approach (open surgery, laparoscopic surgery, conversion to open surgery; conversion to open surgery was defined as any incision made earlier than planned), and operation time. Postoperative outcomes included length of postoperative hospital stay and postoperative complications (Clavien-Dindo classification [12]). All specimens removed during surgery were submitted to the pathology department for macroscopic and microscopic examination, pathologic findings included the presence of ectopic tissue within the MD, and the length and base width of the MD.
We classified our patients into three age groups based on the age at the time of surgery: child group (age of <10 years), adolescent group (age of 10–19 years), and adult group (age of ≥20 years). The age cutoffs we used for each group were based on the World Health Organization’s definition of adolescence. By using these age cutoffs, we were able to analyze the clinical characteristics and treatment outcomes of symptomatic MD in each age group separately.
Data of categorical variables are presented as number of patients and percentages and were compared using the chi-square test or Fisher exact test. Data of continuous variables are presented as mean and standard deviation or as median and interquartile range and were compared using the Kruskal-Wallis test. Data were analyzed using IBM SPSS Statistics for Windows, ver. 24.0 (IBM Corp.). A P-value of <0.05 was considered to indicate statistical significance.
A total of 85 patients underwent MD surgery during the study period. Among them, 43 had symptomatic MD. The median age of symptomatic MD patients was 13 years (range, 0–85 years); 34 of 43 patients (79.1%) were male (male-to-female ratio, 3.8:1). According to the group definition, 14 patients were included in the child group (mean age, 2.79 ± 2.69 years), 17 patients were included in the adolescent group (mean age, 13.71 ± 2.37 years), and 12 patients were included in the adult group (mean age, 36.42 ± 18.73 years).
Table 1 presents the clinical presentation of symptomatic MD in each age group. Among the three groups, vomiting and intestinal obstruction were found to significantly decrease with age (P = 0.042 and P = 0.001, respectively), whereas hematochezia and GI bleeding showed an increasing trend with age, although not statistically significant (P = 0.064 and P = 0.064, respectively).
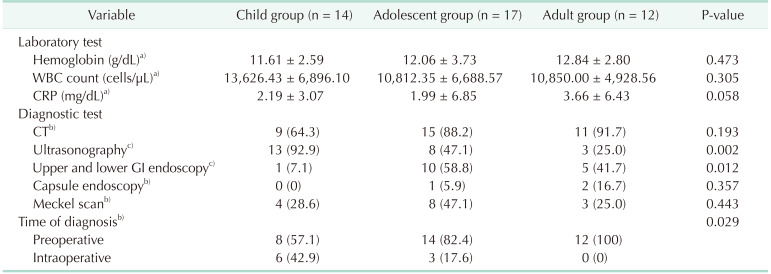
Table 2 shows the performance of different imaging modalities in diagnosing symptomatic MD, stratified by age group. The performance of ultrasound decreased significantly with age (P = 0.002), whereas CT performance showed an increasing trend with age, although not statistically significant (P = 0.193). In patients with GI tract bleeding, upper and lower GI endoscopy (n = 16) was performed on the same day, and capsule endoscopy (n = 3) was performed in patients with obscure GI tract bleeding. However, no patient reported a bleeding focus with endoscopy. Meckel scan (n = 15) was performed in patients with obscure GI tract bleeding. The diagnostic rates of symptomatic MD were found to be higher in CT (71.4%) than in Meckel scan (60.0%) and ultrasound (29.2%). The preoperative diagnosis rate was found to significantly increase with age (P = 0.029).
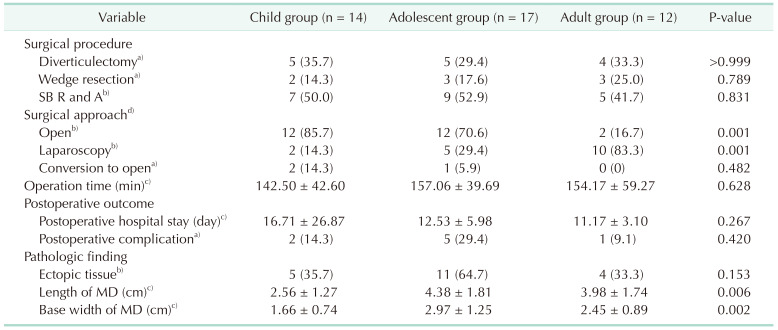
Table 3 presents the operative variables and postoperative outcomes. Small bowel resection and anastomosis were the most commonly performed surgical procedures, followed by diverticulectomy and wedge resection in each age group. Laparoscopic surgery rates increased significantly with age (P = 0.001), but three patients had to undergo conversion from laparoscopy to open surgery due to failed intussusception reduction (n = 1), extended bowel resection (n = 1), and severe inflammatory adhesion (n = 1). The length of postoperative hospital stay did not differ significantly among the age groups (P = 0.267). Eight patients experienced postoperative complications, with seven having Grade I-II complications (Clavien-Dindo classification) such as wound complications (n = 3) and postoperative ileus (n = 4), while one patient had a grade IIIb complication in the form of prolonged postoperative ileus that required reoperation. There was no mortality in the postoperative period.
Ectopic tissues such as gastric and pancreatic mucosa were found twice as much in the adolescent group compared to the other groups, although the difference was not statistically significant (P = 0.153). However, the length and base width of the MD were significantly greater in the adolescent group compared to the other groups (P = 0.006 and P = 0.002, respectively).
MD is the most common congenital anomaly of the GI tract, resulting from incomplete obliteration of the vitelline duct during the fifth week of gestation. Previous studies have reported that the prevalence of MD in the general population is between 1% and 2% [123], with a lifetime risk of symptomatic MD ranging from 4.2% to 9.0% [378]. The most common clinical presentations of symptomatic MD are diverticulitis, intestinal obstruction, and GI tract bleeding. In line with previous studies, our study showed that 44.2%, 27.9%, and 27.9% of patients presented with diverticulitis, intestinal obstruction, and GI tract bleeding, respectively.
Several retrospective studies have investigated the relationship between age and clinical presentations in symptomatic MD, but no conclusive result have been drawn. For example, a single-center retrospective study by Park et al. [6] defined pediatric patients as those younger than 11 years and found that younger patients, especially those younger than 4 years, tended to present with obstruction, whereas older patients tended to present with bleeding. Another single-center retrospective study by Chen et al. [9] defined pediatric patients as those younger than 18 years and reported that GI tract bleeding and obstruction were the most common presentations in pediatric patients, and inflammation was common in adult patients. In our study, symptomatic MD presented as diverticulitis and intestinal obstruction in the child group, whereas it presented as diverticulitis and GI tract bleeding in the adult group. In the adolescent group, we observed diverticulitis (35.3%) and intestinal obstruction (29.4%), which are common in the child group, as well as GI tract bleeding (41.2%), which is common in the adult group. Our results were consistent with previous studies, depending on whether the adolescent group was included in the child group or the adult group. This suggests that the adolescent group had characteristics of both the child group and the adult group.
Preoperative diagnosis of symptomatic MD can be challenging [13]. Kusumoto et al. [14] reported an 88% preoperative diagnosis rate in patients with ulcer bleeding, but only 11% in patients with other complications out of a total of 776 patients. In another retrospective study of 37 patients, Parvanescu et al. [10] reported that only 40% of the patients had a diagnosis of MD. In our study, the preoperative diagnosis rate was 72.1%, which was higher than in previous studies. This was likely due to our high rate of CT scans, which are reliable in detecting symptomatic MD, particularly diverticulitis [1516]. However, whereas USG performed more in the child group than in the other groups to avoid radiation exposure, the preoperative diagnosis rate in the child group was lower than in the other groups. This result was attributed to the fact that the child group had a larger number of intestinal obstructions, which made it more difficult to detect MD using USG.
In addition to USG, endoscopy and capsule endoscopy have shown disappointing results in detecting MD or bleeding focus in patients with GI tract bleeding. Although double-balloon enteroscopy and capsule endoscopy have been reported as effective modalities for diagnosing MD in patients with obscure GI tract bleeding [1718], our endoscopy was not a double-balloon enteroscopy, and capsule endoscopy was performed in only two patients. On the contrary, Meckel scan diagnosed nine patients with obscure GI tract bleeding in our study, and seven of them were not diagnosed using other modalities. Meckel scan is a reliable tool to evaluate patients with obscure GI tract bleeding and has a high diagnostic accuracy for detecting symptomatic MD [19]. It is often difficult to detect MD through imaging studies and endoscopy alone, and clinical suspicion of the attending physician is crucial in diagnosing symptomatic MD.
The treatment for symptomatic MD typically involves surgical resection of the diverticulum or segment of the ileum bearing the diverticulum. Surgical procedure (diverticulectomy, wedge resection, small bowel resection, and anastomosis) is determined by intraoperative findings such as the presence and location of ectopic tissue, and the integrity of the diverticulum base and the adjacent ileum. However, there is no consensus on the optimal surgical procedure for symptomatic MD [20]. In our study, the most common surgical procedure was small bowel resection and anastomosis, which allowed for complete resection of the diverticulum with ectopic tissue and minimized the risk of subsequent luminal narrowing. Laparoscopic surgery has been shown to be safe and feasible for the diagnosis and treatment of symptomatic MD in both pediatric and adult patients [2021222324]. However, our data showed that the rate of laparoscopic surgery increased significantly with age, this was likely due to the fact that the child and adolescent groups presented with more cases of intestinal obstruction, which can make it difficult to create the pneumoperitoneum required for laparoscopic surgery. With the exception of the child group and adolescent group with intestinal obstruction, our results indicate that laparoscopic surgery is a safe and feasible option for the treatment of symptomatic MD.
MD often contains ectopic tissue such as gastric or pancreatic tissue, and symptomatic MD is more likely to have ectopic tissue than asymptomatic MD [6112526]. In our study, ectopic tissue was found in 46.5% of all resected MD specimens, which is consistent with previous studies. It is recommended to remove an MD larger than 2 cm in length, even if it is asymptomatic, as it is a risk factor for developing symptomatic MD [6132027]. Slívová et al. [28] also reported that an MD base width of ≥1.5 cm is associated with a significantly increased risk of gastric heterotopia. In our study, the adolescent group had significantly longer and wider MDs than the other groups, but all groups had MDs that were greater than 2 cm in length and 1.5 cm in base width. Although the adolescent group had a higher prevalence of ectopic tissue within their MDs and larger MD sizes compared to the other groups, further studies with larger sample sizes are needed to confirm the relationship between the presence of ectopic tissue and the size of MD.
This study had several limitations. Firstly, it was a single-center retrospective study with small sample size, as symptomatic MD has a low prevalence in the general population. Secondly, the data collected was limited to what was available in the medical records. Although we used a standardized form consisting of historical and physical examination variables, the data might not be complete or accurate. Finally, the study lacked a control group, so our findings are presented only as absolute numbers and percentages, and we cannot comment on the sensitivity, specificity, or positive and negative predictive values of our results.
In conclusion, our study revealed that symptomatic MD in the adolescent group exhibits a transitional pattern between the child and adult groups in terms of clinical presentations. Whereas diverticulitis and intestinal obstruction are more common in the child group, and diverticulitis and GI tract bleeding are more common in the adult group, the adolescent group presents with these presentations evenly. Furthermore, preoperative diagnosis and laparoscopic surgery rates increase with age, and the adolescent group falls in the middle of these results. Therefore, it is important for clinicians to recognize that adolescent patients with symptomatic MD have characteristics of both child and adult patients to ensure optimal care. We hope that this study will aid in the appropriate diagnosis and treatment of symptomatic MD in patients of various ages and with varying symptoms.
References
1. Haber JJ. Meckel’s diverticulum; review of literature and analytical study of 23 cases with particular emphasis on bowel obstruction. Am J Surg. 1947; 73:468–485. PMID: 20290582.
2. Zani A, Eaton S, Rees CM, Pierro A. Incidentally detected Meckel diverticulum: to resect or not to resect? Ann Surg. 2008; 247:276–281. PMID: 18216533.
3. Hansen CC, Søreide K. Systematic review of epidemiology, presentation, and management of Meckel’s diverticulum in the 21st century. Medicine (Baltimore). 2018; 97:e12154. PMID: 30170459.

4. Weinstein EC, Cain JC, Remine WH. Meckel’s diverticulum: 55 years of clinical and surgical experience. JAMA. 1962; 182:251–253. PMID: 13999637.
5. Meckel JF. Über die divertikel am darmkanal. Arch Physiol. 1809; 9:421–453.
6. Park JJ, Wolff BG, Tollefson MK, Walsh EE, Larson DR. Meckel diverticulum: the Mayo Clinic experience with 1476 patients (1950-2002). Ann Surg. 2005; 241:529–533. PMID: 15729078.
7. Soltero MJ, Bill AH. The natural history of Meckel’s diverticulum and its relation to incidental removal: a study of 202 cases of diseased Meckel’s diverticulum found in King County, Washington, over a fifteen year period. Am J Surg. 1976; 132:168–173. PMID: 952346.

8. Cullen JJ, Kelly KA, Moir CR, Hodge DO, Zinsmeister AR, Melton LJ 3rd. Surgical management of Meckel’s diverticulum: an epidemiologic, population-based study. Ann Surg. 1994; 220:564–569. PMID: 7944666.

9. Chen JJ, Lee HC, Yeung CY, Chan WT, Jiang CB, Sheu JC, et al. Meckel’s diverticulum: factors associated with clinical manifestations. ISRN Gastroenterol. 2014; 2014:390869. PMID: 25006469.

10. Parvanescu A, Bruzzi M, Voron T, Tilly C, Zinzindohoué F, Chevallier JM, et al. Complicated Meckel’s diverticulum: presentation modes in adults. Medicine (Baltimore). 2018; 97:e12457. PMID: 30235734.
11. Bani-Hani KE, Shatnawi NJ. Meckel’s diverticulum: comparison of incidental and symptomatic cases. World J Surg. 2004; 28:917–920. PMID: 15593467.

12. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250:187–196. PMID: 19638912.
13. Sagar J, Kumar V, Shah DK. Meckel’s diverticulum: a systematic review. J R Soc Med. 2006; 99:501–505. PMID: 17021300.

14. Kusumoto H, Yoshida M, Takahashi I, Anai H, Maehara Y, Sugimachi K. Complications and diagnosis of Meckel’s diverticulum in 776 patients. Am J Surg. 1992; 164:382–383. PMID: 1415948.

15. Bennett GL, Birnbaum BA, Balthazar EJ. CT of Meckel’s diverticulitis in 11 patients. AJR Am J Roentgenol. 2004; 182:625–629. PMID: 14975960.

16. Kawamoto S, Raman SP, Blackford A, Hruban RH, Fishman EK. CT detection of symptomatic and asymptomatic Meckel diverticulum. AJR Am J Roentgenol. 2015; 205:281–291. PMID: 26204277.

17. He Q, Zhang YL, Xiao B, Jiang B, Bai Y, Zhi FC. Double-balloon enteroscopy for diagnosis of Meckel’s diverticulum: comparison with operative findings and capsule endoscopy. Surgery. 2013; 153:549–554. PMID: 23305600.

18. Krstic SN, Martinov JB, Sokic-Milutinovic AD, Milosavljevic TN, Krstic MN. Capsule endoscopy is useful diagnostic tool for diagnosing Meckel’s diverticulum. Eur J Gastroenterol Hepatol. 2016; 28:702–707. PMID: 26854797.

19. Suh M, Lee HY, Jung K, Kim SE. Diagnostic accuracy of meckel scan with initial hemoglobin level to detect symptomatic Meckel diverticulum. Eur J Pediatr Surg. 2015; 25:449–453. PMID: 25422901.

20. Blouhos K, Boulas KA, Tsalis K, Barettas N, Paraskeva A, Kariotis I, et al. Meckel’s diverticulum in adults: surgical concerns. Front Surg. 2018; 5:55. PMID: 30234126.

21. Shalaby RY, Soliman SM, Fawy M, Samaha A. Laparoscopic management of Meckel’s diverticulum in children. J Pediatr Surg. 2005; 40:562–567. PMID: 15793736.

22. Sharma RK, Jain VK. Emergency surgery for Meckel’s diverticulum. World J Emerg Surg. 2008; 3:27. PMID: 18700974.

23. Hosn MA, Lakis M, Faraj W, Khoury G, Diba S. Laparoscopic approach to symptomatic Meckel diverticulum in adults. JSLS. 2014; 18:e2014.00349.

24. Jung HS, Park JH, Yoon SN, Kang BM, Oh BY, Kim JW. Clinical outcomes of minimally invasive surgery for Meckel diverticulum: a multicenter study. Ann Surg Treat Res. 2020; 99:213–220. PMID: 33029480.

25. Burjonrappa S, Khaing P. Meckel’s diverticulum and ectopic epithelium: evaluation of a complex relationship. J Indian Assoc Pediatr Surg. 2014; 19:85–89. PMID: 24741211.

26. Lohsiriwat V, Sirivech T, Laohapensang M, Pongpaibul A. Comparative study on the characteristics of Meckel’s diverticulum removal from asymptomatic and symptomatic patients: 18-year experience from Thailand’s largest university hospital. J Med Assoc Thai. 2014; 97:506–512. PMID: 25065089.
27. Mackey WC, Dineen P. A fifty year experience with Meckel’s diverticulum. Surg Gynecol Obstet. 1983; 156:56–64. PMID: 6600203.
28. Slívová I, Vávrová Z, Tomášková H, Okantey O, Penka I, Ihnát P. Meckel’s diverticulum in children: parameters predicting the presence of gastric heterotopia. World J Surg. 2018; 42:3779–3784. PMID: 29750325.

Table 1
Comparison of demographic information and clinical presentations among groups
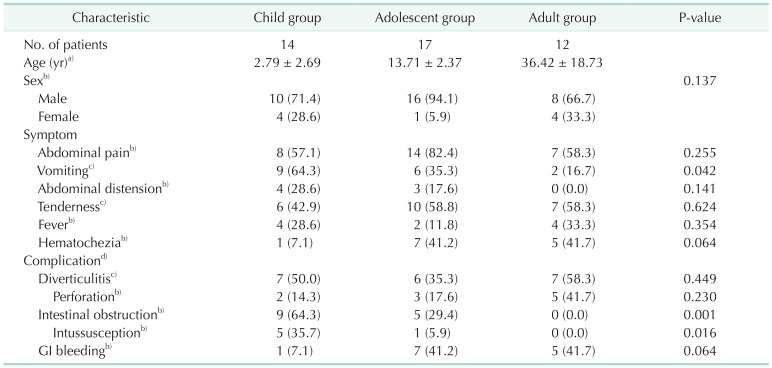
Values are presented as number only, mean ± standard deviation, or number (%).
GI, gastrointestinal.
a)Kruskal-Wallis test, b)Fisher exact test, and c)chi-square test. d)One or more complications are listed; perforation and intussusception are subgroups of diverticulitis and intestinal obstruction, respectively.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download