Abstract
Although the epiglottis plays a vital role in deglutition, histological studies of the epiglottis and surrounding ligaments associated with swallowing dysfunction are limited. Therefore, we performed histological observations to clarify age-related changes in the morphological characteristics of the epiglottis and surrounding structures. Tissue samples comprising the epiglottis and surrounding structures were collected from corpses that were both orally fed and tube-fed during their lifetimes. Following hematoxylin and eosin, Elastica Van Gieson, and immunohistochemical staining procedures, the chondrocytes, connective tissue, and glandular tissue were observed under the epiglottis epithelium, and intervening adipose tissue was observed in the surrounding area. Fatty degeneration of acinar cells was also observed in the glandular tissue, possibly because of aging. Bundles of elastic fibers were present around the vascular wall in the peri-epiglottic ligament, but some were reduced. Furthermore, large amounts of collagen fibers ran toward and through the cartilage, whereas the mesh-like elastic fibers stopped in front of the cartilage. Microfibrils considered to be oxytalan fibers, which are thinner and shorter than elastic fibers, were observed around the vascular wall and in the fiber bundles. Age-related changes included connective tissue fibrosis shown by the large amount of collagen fibers, atrophy of salivary glands, and an accompanying increase in adipose tissue. Regarding stretchability and elasticity, the elastic fibers may have an auxiliary function for laryngeal elevation during deglutition. This suggests that disuse atrophy of the laryngeal organs with or without oral intake might reduce the amount of elastic fiber in older adults.
The series of movements involved in deglutition are coordinated by the larynx and surrounding organs. The epiglottis plays a particularly important role in this process, preventing aspiration caused by food by collapsing and blocking the airway in the series of swallowing processes [1]. The deglutition function declines because of various factors associated with aging [2]; therefore, movement of the epiglottis, hyoid bone, and surrounding muscles during deglutition may also be affected by aging.
The risk of laryngeal penetration with a straw during deglutition movements increases in older adults compared to young people, even in healthy subjects [3]. One of the reasons for this change may be that the height between the hyoid bone and the larynx increases with age, and the maximum expanded width of the pharynx increases [4]. Furthermore, in the hyoid–laryngeal organ system, which includes the hyoid bone and epiglottis, the vertical distance traveled during normal swallowing is longer in people over 65 years of age than in young people [5]. In other words, the laryngeal elevation is insufficient in older adults because of the low position of the larynx, which prevents the epiglottis from descending and causes aspiration.
During deglutition, the larynx elevates under the action of the suprahyoid and infrahyoid muscles. As a result, the force pushing up the airway puts pressure on the base of the tongue, causing the epiglottis to descend and close the laryngeal opening, blocking the airway [6]. Thus, the tongue is an important organ that controls the correct movement of the epiglottis. As tongue movement is synchronous and periodic with movement of the jaw and hyoid bone, mastication and deglutition are impaired if effective tongue movement does not occur [7]. Therefore, the known reduction in tongue pressure when swallowing food among older adults [8] can lead to insufficient descent of the epiglottis and subsequent aspiration.
Furthermore, three-dimensional computed tomography (CT) images have indicated that the length and volume of the pharynx and larynx are affected by age and gender, being significantly larger in males than females and in older adults than younger adults [9]. The position of the larynx is thought to lower with age-related changes, resulting in insufficient laryngeal elevation, which leads to aspiration [4, 5]. The pharyngeal phase of reflex swallowing is the phase of deglutition that is directly related to aspiration [7]. Therefore, it is important to have a precise understanding of the epiglottal movement that prevents bolus entry into the trachea.
Although many studies have investigated age-related changes in the larynx, with a focus on the vocal cords [10, 11], few have analyzed age-related changes in the epiglottis and surrounding structures from a histological point of view. Therefore, in this study, we conduct histological observations through hematoxylin and eosin staining, connective tissue staining, and immunohistochemical staining to clarify morphological changes in the epiglottis and surrounding structures of older adults, including the route and distribution of fibers and blood vessels.
A total of 20 human subjects (12 males and 8 females) donated to Ohu University were analyzed in this study. Two subjects (a male and a female) had experienced gastrostomy and were assumed to have had no oral intake for a period of time. The ages of all subjects ranged from 64 to 94, with an average age of 79.8. This study was approved by the Ethics Committee of Ohu University (Receipt number: No. 173). Dissections were fixed by arterial perfusion with 10% formalin solution and stored in 60% ethanol solution. Samples containing the epiglottis and surrounding structures, including the thyroid cartilage and hyoid bone, were collected from each dissected body and used as observation regions (Fig. 1).
After fixing the samples with 4% paraformaldehyde-phosphate buffer (Nacalai Tesque) for one day, the samples were then decalcified with 0.5 mol/L ethylene-diamine-tetraacetic acid solution (Wako) or Planck-Ryuclo solution (Muto) for 14–30 days. Thereafter, samples were embedded in paraffin, and a sagittal slice was prepared with a thickness of approximately 5 μm. The samples were then subjected to hematoxylin and eosin staining to observe the whole image, Elastica Van Gieson staining to observe the distribution and route of fibers, and immunohistochemical staining.
The primary antibodies for immunohistochemical staining were Polyclonal Rabbit Anti-Human Von Willebrand Factor (1:100; Dako) and Polyclonal Rabbit Anti-Fibrillin-1 antibody (1:100; Abcam). Antigen retrieval was performed with 25X Proteinase K (Diagnostic BioSystems). The secondary antibody was labeled with HRP, and the antigen-antibody reaction was detected by diaminobenzidine and HRP catalytic reaction. In addition, some samples were stained using an avidin/biotin-based enzyme amplification system (VECTASTAIN® Elite® ABC-HRP Kit; Vector) to obtain clearer and more sensitive specific staining images. The antigen-antibody reaction was visualized by the 3,3’ Diaminobenzidine method. Counterstaining with methyl green (Vector) was performed on the same samples. Observations were performed using an Axio Vison Imager M2 (Carl Zeiss).
Underneath the epithelium of the epiglottis, tissues including cartilaginous tissue, connective tissue, and glandular tissue were observed, with intervening adipose tissue surrounding these tissues. In some glandular tissue, fatty degeneration of acinar cells was observed, which is thought to be due to aging (Fig. 2). Around the thyroepiglottic ligament, bundles of elastic fibers were abundant around the blood vessel wall. In this ligament, collagen fibers exhibited a straight morphology that extended toward the cartilage. Conversely, the abundant elastic fibers were distributed in a mesh-like pattern, forming a crosslinked structure that discontinued in front of the cartilage. In this experiment, collagen fibers were stained red, and elastic fibers were stained black-purple (Fig. 3). Both collagen fibers and elastic fibers were observed in the hyoid-epiglottic ligament, running along the perichondrium of the epiglottis and connecting with each other. A certain amount of elastic fibers were present near the epiglottis, and many were found near the center of the ligament. However, almost no elastic fibers were observed toward the hyoid bone, whereas collagen fibers were abundant (Fig. 4). Additionally, some samples contained abundant collagen fibers but few elastic fibers (Fig. 5).
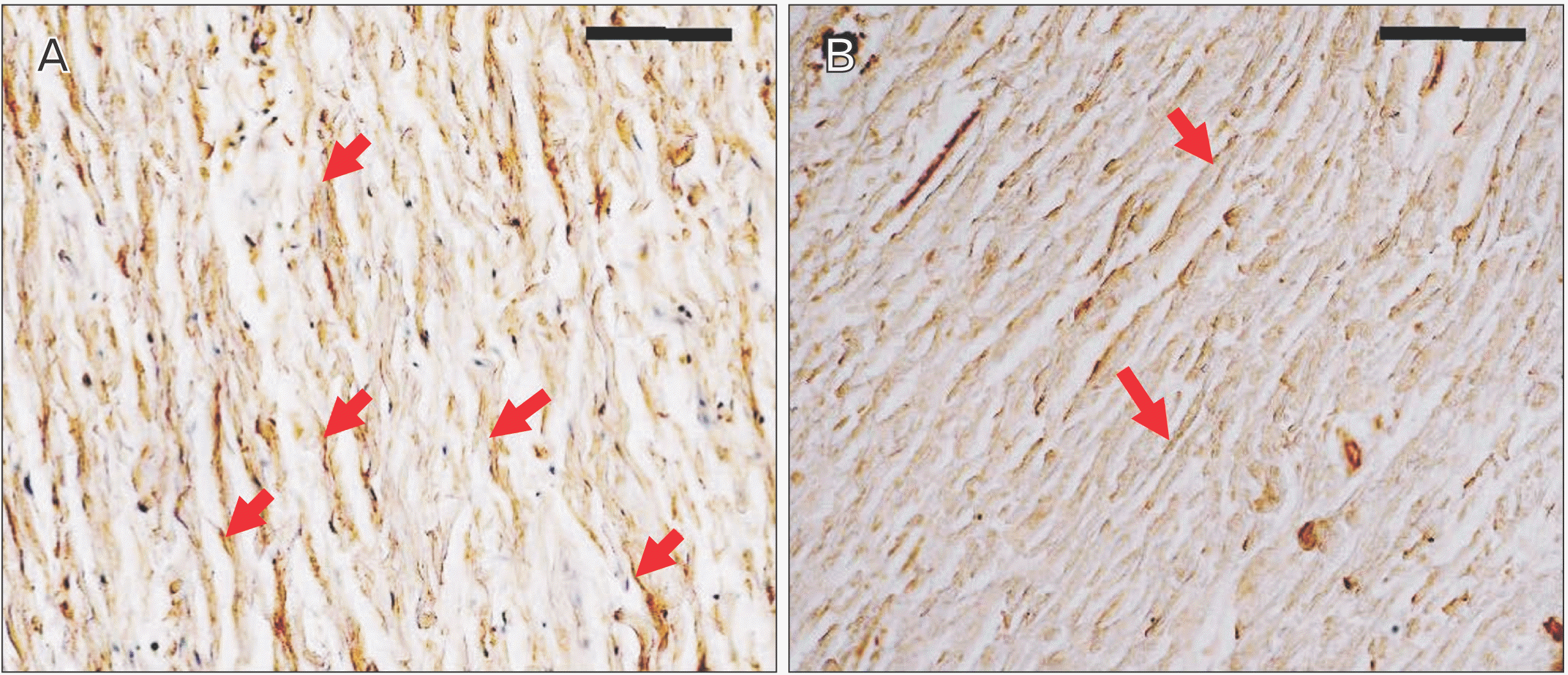
Immunohistochemical staining of factor VIII showed the distribution of blood vessels under the epiglottis and in the fiber bundles of the surrounding ligament (Fig. 6). In addition, immunohistochemical staining of fibrillin 1 revealed structures considered to be fibrillin 1 antibody-positive microfibrils, which are thinner and shorter than elastic fibers in the surroundings of the blood vessel walls and in fiber bundles (Fig. 7). According to the immunohistochemical staining image of fibrillin 1 in the thyroepiglottic and hyoid epiglottic ligaments, the number of fibrillin 1 antibody-positive microfibrils was larger in the samples collected from subjects capable of oral intake before death than in those collected from subjects who relied on enteral nutrition (Figs. 8, 9).
The epiglottis is one of the organs that forms the larynx. The regular flat leaf-like structure of the epiglottis collapses backward when swallowing to block the airway and prevent food from entering the larynx [1, 7]. This function is closely related to aspiration in older adults. That is, the deglutition function declines with aging because of functional deterioration causing insufficient backward descent during deglutition, which leads to aspiration [2]. Insufficient epiglottic descent is associated with the following diverse reasons, among others: an increase in the distance between the hyoid bone and the larynx and the width of the maximally dilated pharynx [4], insufficient laryngeal elevation caused by increased vertical movement of the larynx during deglutition [5], and decreased tongue pressure during deglutition [8]. In addition, the epiglottis itself is also affected by aging in the same way as other organs of the body, experiencing changes such as tissue atrophy [12] and increased calcification of bone-like cartilage [13].
Aging is generally associated with changes in body composition, such as bone and muscle loss and fat gain in the human body [14]. The observed changes such as atrophy of the glandular tissue observed under the epiglottis and an accompanying increase in adipose tissue are thought to be caused by aging. Moreover, peripheral vascular resistance also increases with aging, whereas cardiac output and tissue and organ perfusion decrease [15]. In this study, blood vessels were observed in the epiglottis and surrounding tissues; however, it is possible that blood flow to the tissues was reduced by the effects of aging.
In general, ligaments present in living organisms contain collagen fibers that give strength and tension to tissues and elastic fibers that give stretchability and elasticity to tissues as extracellular matrices [16]. From our observations, we confirmed the presence of collagen fibers and elastic fibers in the ligament of the epiglottis. We also observed sites of connective tissue with a large amount of collagen fibers considered to be fibrotic. In addition, some specimens contained abundant collagen fibers but little to no elastic fibers.
An increase in collagen fibers and fragmentation of elastic fibers were reported to be observed in the fibrous structure of heart valves due to aging [17]. Lumbar spinal canal stenosis, a typical spinal disease among older adults, is reportedly caused by thickening of the ligamentum flavum, characterized by increased proteoglycan, which is thought to inhibit the aggregation of elastic fibers, and reduced elastic fibers [18]. The collagen and elastin content of the hyoid–epiglottic ligament also decreases with aging [19]. Furthermore, the number of elastic fibers is significantly lower in older adults than in young people [20]. In the normal epiglottis, the dominant function of collagen fibers is mechanical strength, whereas the elastic fibers play an auxiliary role by affording stretchability and elasticity when the larynx descends during deglutition. Thus, the observed increase in collagen fibers and decrease in elastic fibers in this study are considered to be changes caused by aging. These changes may affect the deglutition function of older adults through a decrease in stretchability and elasticity and insufficient epiglottic descent during deglutition.
Fibers named oxytalan fibers (hereinafter Ox) are included in the elastic fiber system, but exist as bundles of microfibril lining the basement membrane of the epithelium, independently of mature elastic fibers [21]. Ox is present in sites subject to mechanical stress, such as the skin, tendons, ligaments, joints, and tunica adventitia, suggesting that Ox is involved in maintaining the vascular system, regulating blood flow, and contributing to the mechanical properties of ligaments and tendons [22-25]. The amount of Ox decreases with aging in human and rat tissues [26, 27]. In addition, age-related changes in the elastic fiber system of the interfoveolar ligament in the groin showed an increase in mature elastic fibers. Moreover, Ox decreases progressively, thereby inducing a loss of ligament elasticity [28]. Similarly, comparing the composition of fibrous components of the spinous ligament of the cervical vertebrae between young and old people revealed that collagen fibers increased with aging, whereas Ox decreased [26].
Immunohistochemical staining revealed fibrillin-1 antibody-positive microfiber structures, which are the main constituent molecules of Ox, around the vascular walls and in the fiber bundles distributed in the epiglottis [29]. These structures are considered to be Ox, and exhibited a finer and shorter structure than elastic fibers. Comparing the samples from corpses that were capable of oral intake before death with those from corpses who relied on enteral nutrition, the amount of Ox was lower in the latter group (Figs. 8, 9). Elderly people with systemic diseases or who are bedridden are at risk of malnutrition and rely on enteral nutrition to manage their nutritional status if they cannot take food orally [30, 31]. However, long-term rest and non-use of systemic organs can cause disuse atrophy because of reduced mechanical stress [32, 33]. The epiglottis is also connected to the surrounding cartilage by ligaments, and is one of the organs that is constantly exposed to mechanical stress during movement to prevent the bolus from entering the trachea during swallowing. However, in older adults who rely on enteral nutrition, the need for swallowing movements disappears, leading to disuse atrophy around the larynx. This suggests that the progression of disuse atrophy may influence the amount of Ox involved in the mechanical properties of the epiglottis and surrounding ligaments.
Notes
References
1. Lamb J. 1974; Movements and functions of the epiglottis during deglutition. Edinb Dent Hosp Gaz. 13:22–30. PMID: 4532072.
2. Gleeson DC. 1999; Oropharyngeal swallowing and aging: a review. J Commun Disord. 32:373–95. quiz 395–6. DOI: 10.1016/S0021-9924(99)00017-9. PMID: 10560713.
3. Daniels SK, Corey DM, Hadskey LD, Legendre C, Priestly DH, Rosenbek JC, Foundas AL. 2004; Mechanism of sequential swallowing during straw drinking in healthy young and older adults. J Speech Lang Hear Res. 47:33–45. DOI: 10.1044/1092-4388(2004/004). PMID: 15072526.

4. Leonard R, Kendall KA, McKenzie S. 2004; Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia. 19:133–41. DOI: 10.1007/s00455-003-0508-6. PMID: 15382802.

5. Kang BS, Oh BM, Kim IS, Chung SG, Kim SJ, Han TR. 2010; Influence of aging on movement of the hyoid bone and epiglottis during normal swallowing: a motion analysis. Gerontology. 56:474–82. DOI: 10.1159/000274517. PMID: 20068282.

6. Satoda T, Shimoe S, Makihira S, Tamamoto M, Murayama T, Nikawa H. 2008; Model for the functional instruction of swallowing. Kaibogaku Zasshi. 83:51–7. Japanese. PMID: 18572803.
7. Standring S. 2016. Gray's anatomy: the anatomical basis of clinical practice. 41st ed. Elsevier;p. 583.
8. Tamine K, Ono T, Hori K, Kondoh J, Hamanaka S, Maeda Y. 2010; Age-related changes in tongue pressure during swallowing. J Dent Res. 89:1097–101. DOI: 10.1177/0022034510370801. PMID: 20530725.

9. Inamoto Y, Saitoh E, Okada S, Kagaya H, Shibata S, Baba M, Onogi K, Hashimoto S, Katada K, Wattanapan P, Palmer JB. 2015; Anatomy of the larynx and pharynx: effects of age, gender and height revealed by multidetector computed tomography. J Oral Rehabil. 42:670–7. DOI: 10.1111/joor.12298. PMID: 25892610.

10. Ximenes Filho JA, Tsuji DH, do Nascimento PH, Sennes LU. 2003; Histologic changes in human vocal folds correlated with aging: a histomorphometric study. Ann Otol Rhinol Laryngol. 112:894–8. DOI: 10.1177/000348940311201012. PMID: 14587982.

11. Kuhn MA. 2014; Histological changes in vocal fold growth and aging. Curr Opin Otolaryngol Head Neck Surg. 22:460–5. DOI: 10.1097/MOO.0000000000000108. PMID: 25232935.

12. Pellnitz D. 1961; On change in the morphology of the human epiglottis due to aging. Arch Ohren Nasen Kehlkopfheilkd. 178:350–4. German. DOI: 10.1007/BF02103223. PMID: 14484990.
13. Kano M, Shimizu Y, Okayama K, Igari T, Kikuchi M. 2005; A morphometric study of age-related changes in adult human epiglottis using quantitative digital analysis of cartilage calcification. Cells Tissues Organs. 180:126–37. DOI: 10.1159/000086753. PMID: 16113541.

14. JafariNasabian P, Inglis JE, Reilly W, Kelly OJ, Ilich JZ. 2017; Aging human body: changes in bone, muscle and body fat with consequent changes in nutrient intake. J Endocrinol. 234:R37–51. DOI: 10.1530/JOE-16-0603. PMID: 28442508.

15. Bender AD. 1965; The effect of increasing age on the distribution of peripheral blood flow in man. J Am Geriatr Soc. 13:192–8. DOI: 10.1111/j.1532-5415.1965.tb02665.x. PMID: 14270624.

16. Henninger HB, Ellis BJ, Scott SA, Weiss JA. 2019; Contributions of elastic fibers, collagen, and extracellular matrix to the multiaxial mechanics of ligament. J Mech Behav Biomed Mater. 99:118–26. DOI: 10.1016/j.jmbbm.2019.07.018. PMID: 31351401. PMCID: PMC7474469.

17. Gumpangseth T, Lekawanvijit S, Mahakkanukrauh P. 2020; Histological assessment of the human heart valves and its relationship with age. Anat Cell Biol. 53:261–71. DOI: 10.5115/acb.20.093. PMID: 32727956. PMCID: PMC7527117.

18. Yabe Y, Hagiwara Y, Tsuchiya M, Honda M, Hatori K, Sonofuchi K, et al. 2016; Decreased elastic fibers and increased proteoglycans in the ligamentum flavum of patients with lumbar spinal canal stenosis. J Orthop Res. 34:1241–7. DOI: 10.1002/jor.23130. PMID: 26679090.

19. Irvine LE, Yang Z, Kezirian EJ, Nimni ME, Han B. 2018; Hyoepiglottic ligament collagen and elastin fiber composition and changes associated with aging. Laryngoscope. 128:1245–8. DOI: 10.1002/lary.27094. PMID: 29330863.

20. Sawatsubashi M, Umezaki T, Kusano K, Tokunaga O, Oda M, Komune S. 2010; Age-related changes in the hyoepiglottic ligament: functional implications based on histopathologic study. Am J Otolaryngol. 31:448–52. DOI: 10.1016/j.amjoto.2009.08.003. PMID: 20015802.

21. Böck P. 1983; Elastic fiber microfibrils: filaments that anchor the epithelium of the epiglottis. Arch Histol Jpn. 46:307–14. DOI: 10.1679/aohc.46.307. PMID: 6685469.

22. Goldfischer S, Coltoff-Schiller B, Schwartz E, Blumenfeld OO. 1983; Ultrastructure and staining properties of aortic microfibrils (oxytalan). J Histochem Cytochem. 31:382–90. DOI: 10.1177/31.3.6186732. PMID: 6186732.

23. Strydom H, Maltha JC, Kuijpers-Jagtman AM, Von den Hoff JW. 2012; The oxytalan fibre network in the periodontium and its possible mechanical function. Arch Oral Biol. 57:1003–11. DOI: 10.1016/j.archoralbio.2012.06.003. PMID: 22784380.

24. Inoue K, Hara Y, Sato T. 2012; Development of the oxytalan fiber system in the rat molar periodontal ligament evaluated by light- and electron-microscopic analyses. Ann Anat. 194:482–8. DOI: 10.1016/j.aanat.2012.03.010. PMID: 22727934.

25. Giusti B, Pepe G. 2016; Fibrillins in tendon. Front Aging Neurosci. 8:237. DOI: 10.3389/fnagi.2016.00237. PMID: 27812333. PMCID: PMC5071311. PMID: 162c0652ed0e41b8a41caeef487c241b.

26. Barros EM, Rodrigues CJ, Rodrigues NR, Oliveira RP, Barros TE, Rodrigues AJ Jr. 2002; Aging of the elastic and collagen fibers in the human cervical interspinous ligaments. Spine J. 2:57–62. DOI: 10.1016/S1529-9430(01)00167-X. PMID: 14588289.

27. Rodrigues CJ, Rodrigues Junior AJ. 2000; A comparative study of aging of the elastic fiber system of the diaphragm and the rectus abdominis muscles in rats. Braz J Med Biol Res. 33:1449–54. DOI: 10.1590/S0100-879X2000001200008. PMID: 11105097. PMID: e778f79cdda84ec6936e12844aad744e.

28. Quintas ML, Rodrigues CJ, Yoo JH, Rodrigues Junior AJ. 2000; Age related changes in the elastic fiber system of the interfoveolar ligament. Rev Hosp Clin Fac Med Sao Paulo. 55:83–6. DOI: 10.1590/S0041-87812000000300003. PMID: 10983010.

29. Ramirez F, Pereira L. 1999; The fibrillins. Int J Biochem Cell Biol. 31:255–9. DOI: 10.1016/S1357-2725(98)00109-5. PMID: 10216958.

30. Finocchiaro E, Galletti R, Costantino A, Rivetti M, Xompero G, Aimonino N, Balzola F. 1992; Enteral nutrition in the elderly. Minerva Gastroenterol Dietol. 38:109–13. Italian. PMID: 1391146.
31. Nogami T, Kurachi M, Hukushi T, Iwasaki K. 2020; Recovery of oral feeding in Japanese elderly people after long-term tube feeding: a challenge in Miyama Hospital. J Family Med Prim Care. 9:3977–80. DOI: 10.4103/jfmpc.jfmpc_567_20. PMID: 33110796. PMCID: PMC7586527. PMID: b6969d91465847d1a381644d287b272f.

32. Katsume H, Furukawa K, Azuma A, Nakamura T, Matsubara K, Ohnishi K, Sugihara H, Asayama J, Nakagawa M. 1992; Disuse atrophy of the left ventricle in chronically bedridden elderly people. Jpn Circ J. 56:201–6. DOI: 10.1253/jcj.56.201. PMID: 1552647.

33. Wall BT, Dirks ML, van Loon LJ. 2013; Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. 12:898–906. DOI: 10.1016/j.arr.2013.07.003. PMID: 23948422.

Fig. 1
Schematic diagrams of (A) the larynx (sagittal section), the enlarged view of the smaller rectangle corresponds to (B) and the enlarged view of the larger rectangle corresponds to (C). (B) the thyroepiglottic ligament, and (C) the hyoid-epiglottic ligament.
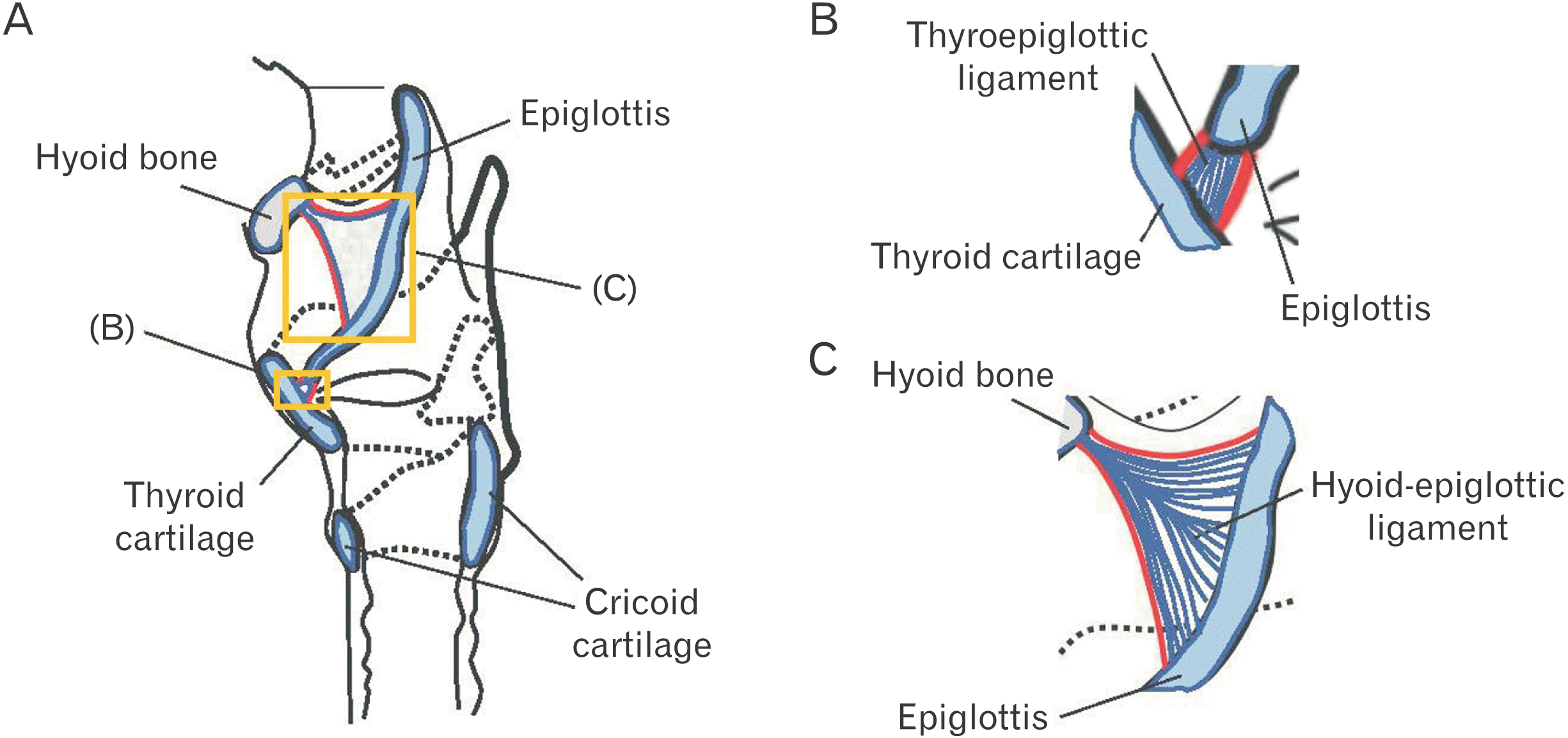
Fig. 2
Hematoxylin & eosin staining image under epiglottis epithelium (×4). (A) and (B) are stained images of different samples. The image in the lower right of (B) is a magnified portion of the glandular tissue (×40). Black scale bar: 200 μm and red scale bar: 100 μm. Red arrowheads indicate acinar tissues (fatty degeneration). AC, acinar cells (normal); BV, blood vessels; C, cartilage; F, fat; P, perichondrium.
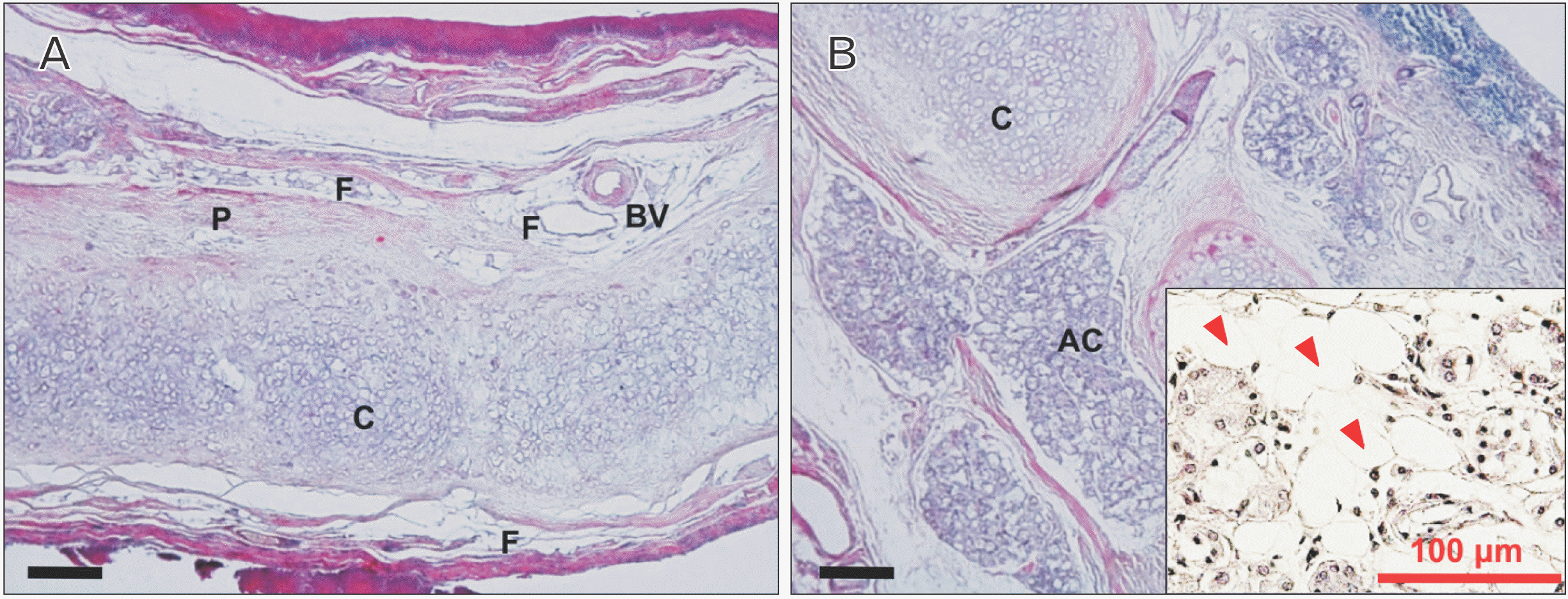
Fig. 3
Elastica Van Gieson stained image near the thyroid cartilage in the thyroepiglottic ligament ([A] ×5, scale bar: 200 μm; [B] ×20, scale bar: 50 μm). Asterisks (*) indicate elastic fibers and arrowheads indicates collagen fibers. TC, thyroid cartilage.
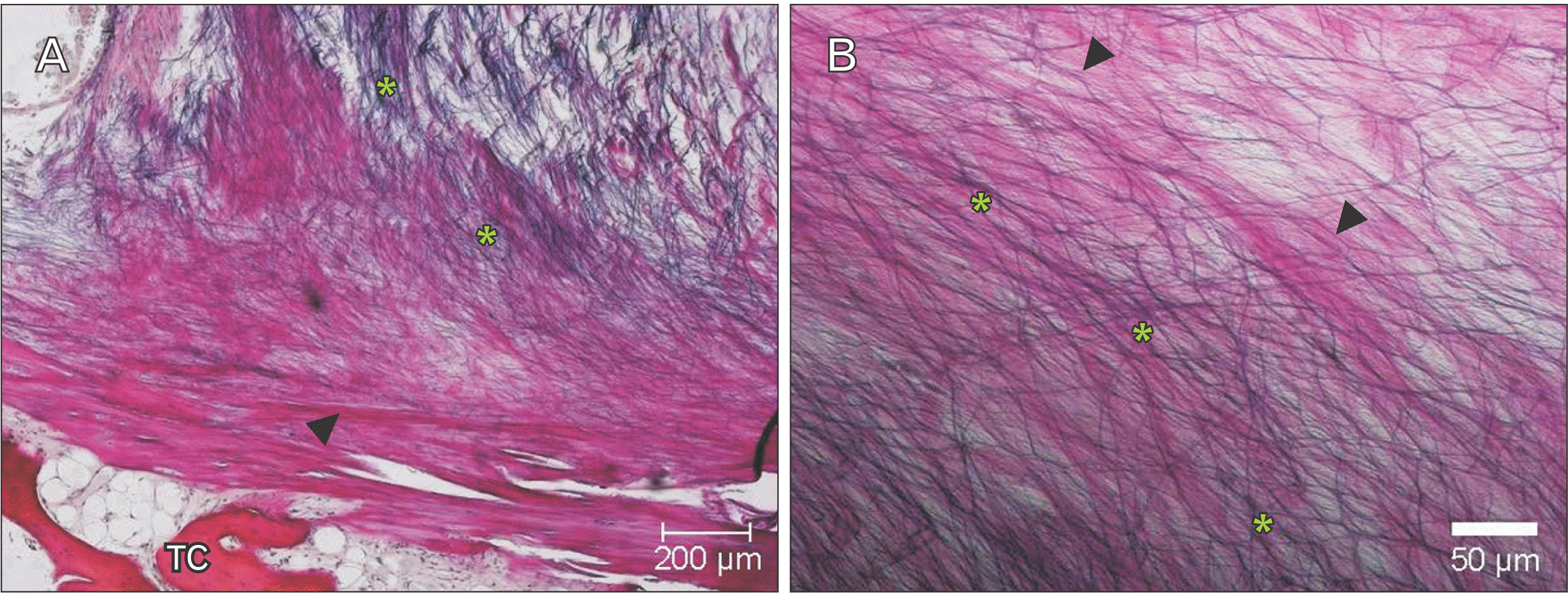
Fig. 4
Elastica Van Gieson stained image of the hyoid–epiglottic ligament. (A) Fiber bundles running along the epiglottis (×10, scale bar: 100 μm), (B) near the hyoid bone (×20, scale bar: 50 μm), (C) near the center of the ligament (×20, scale bar: 50 μm), and (D) near the epiglottis (×20, scale bar: 50 μm). Asterisks (*) indicate elastic fibers and arrowheads indicate collagen fibers. EC, epiglottis; GT, glandular tissue; HB, hyoid bone; P, perichondrium.
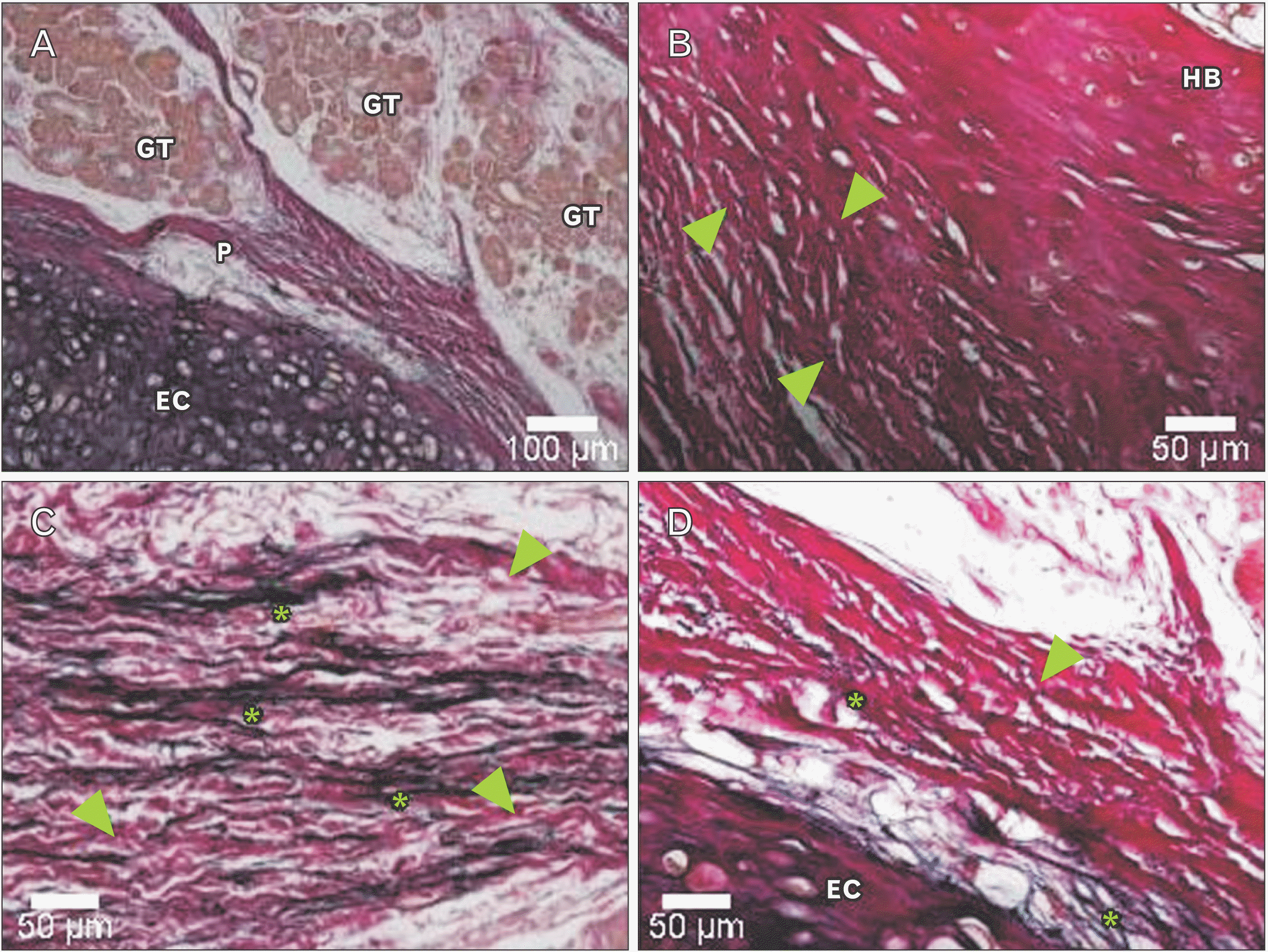
Fig. 5
Elastica Van Gieson staining images of (A) near the hyoid bone (×5, scale bar: 200 μm), (B) enlarged image of the square part of (A) (×20, scale bar: 50 μm), (C) near the epiglottis (×10, scale bar: 100 μm), and (D) near the center of the ligament (×20, scale bar: 50 μm). Arrowheads indicate collagen fiber. BV, blood vessel; EC, epiglottis; GT, glandular tissue; HB, hyoid bone.
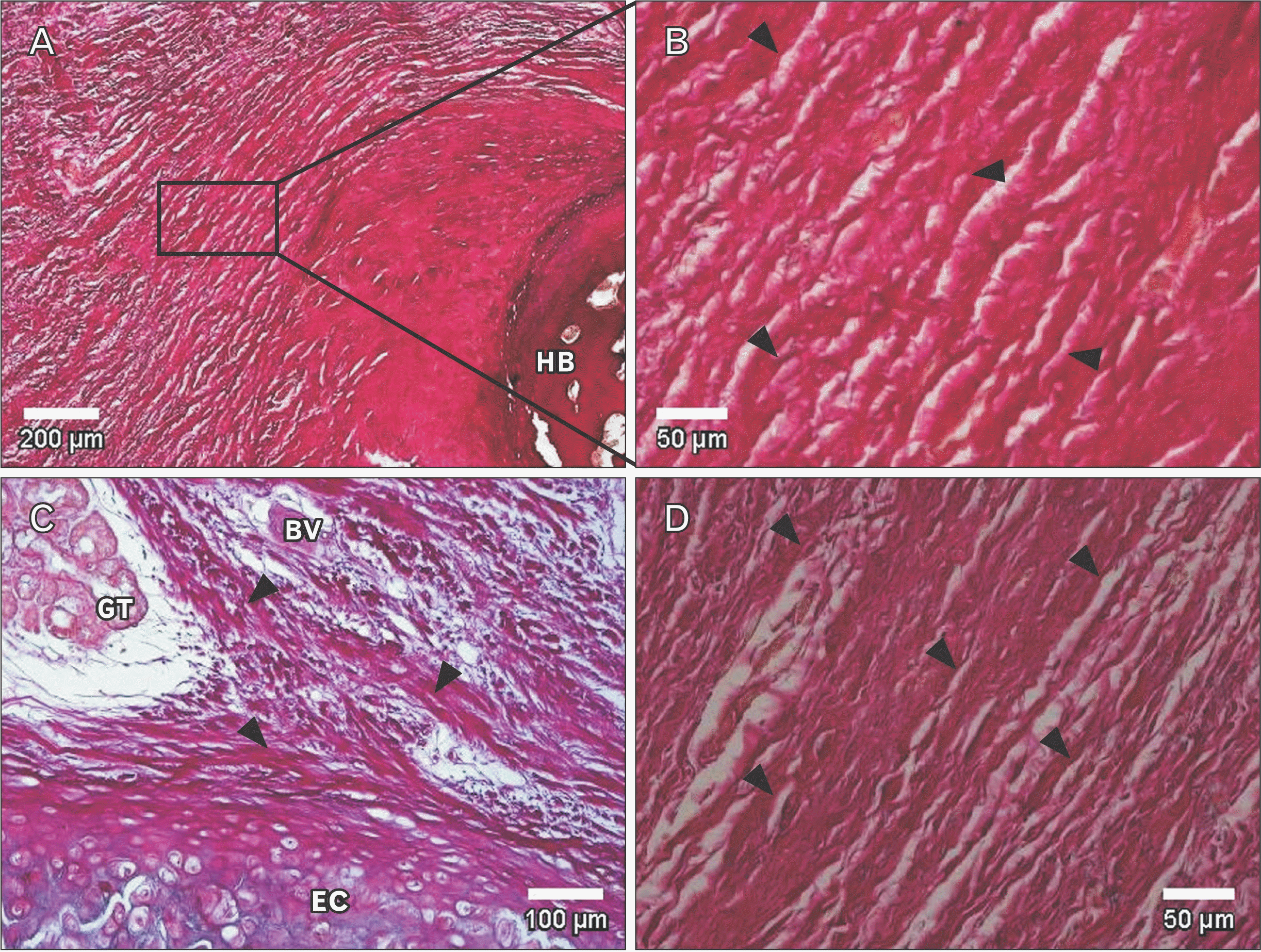
Fig. 6
(A) Immunohistochemical staining of factor VIII near the epiglottis (×20) and (B) immunohistochemical staining of factor VIII in ligamentous fibers (×20). Scale bar: 50 μm. Arrowheads indicate collagen fibers and red arrows indicate capillaries (factor VIII positive). EC, epiglottis.
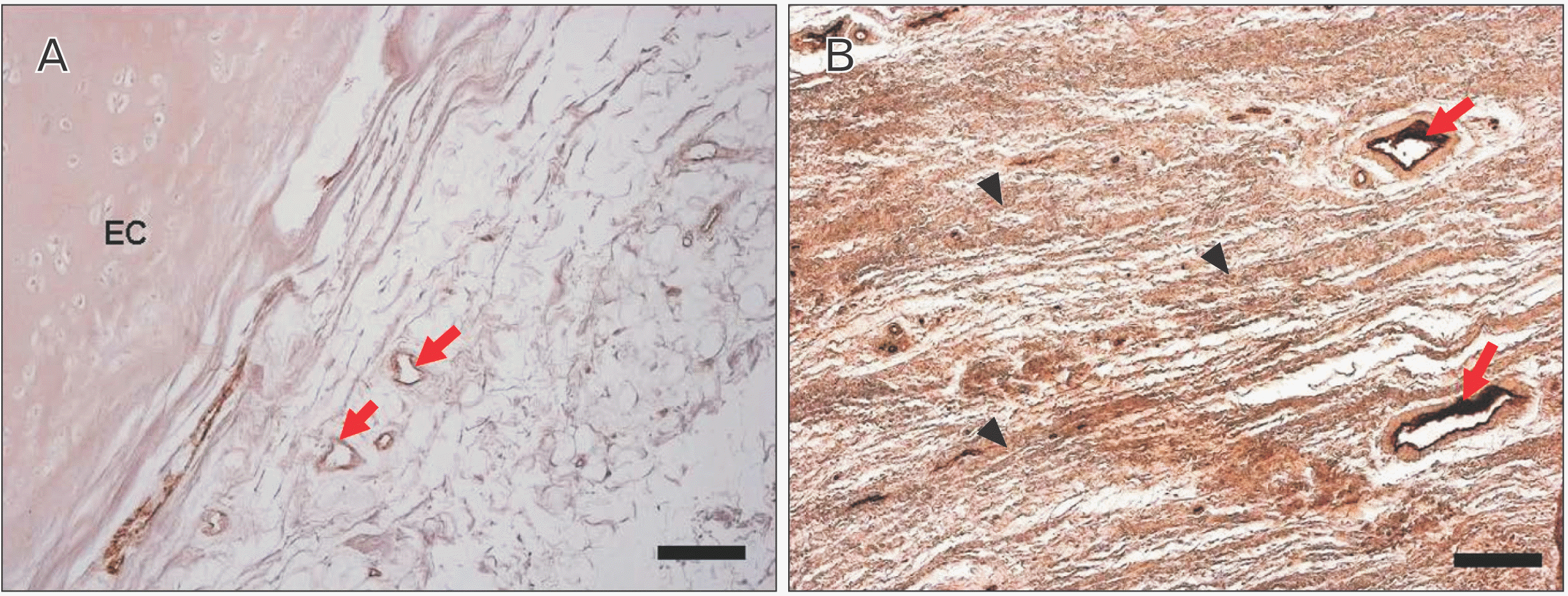
Fig. 7
(A) Immunohistochemical staining image of fibrillin 1 in ligamentous fibers (×20). (B) Immunohistochemical staining image of fibrillin 1 around the blood vessel wall distributed in the epiglottis (×20). Scale bar: 50 μm. Arrowheads indicate collagen fiber and arrows indicate Fibrillin 1 antibody-positive site. BV, blood vessel.
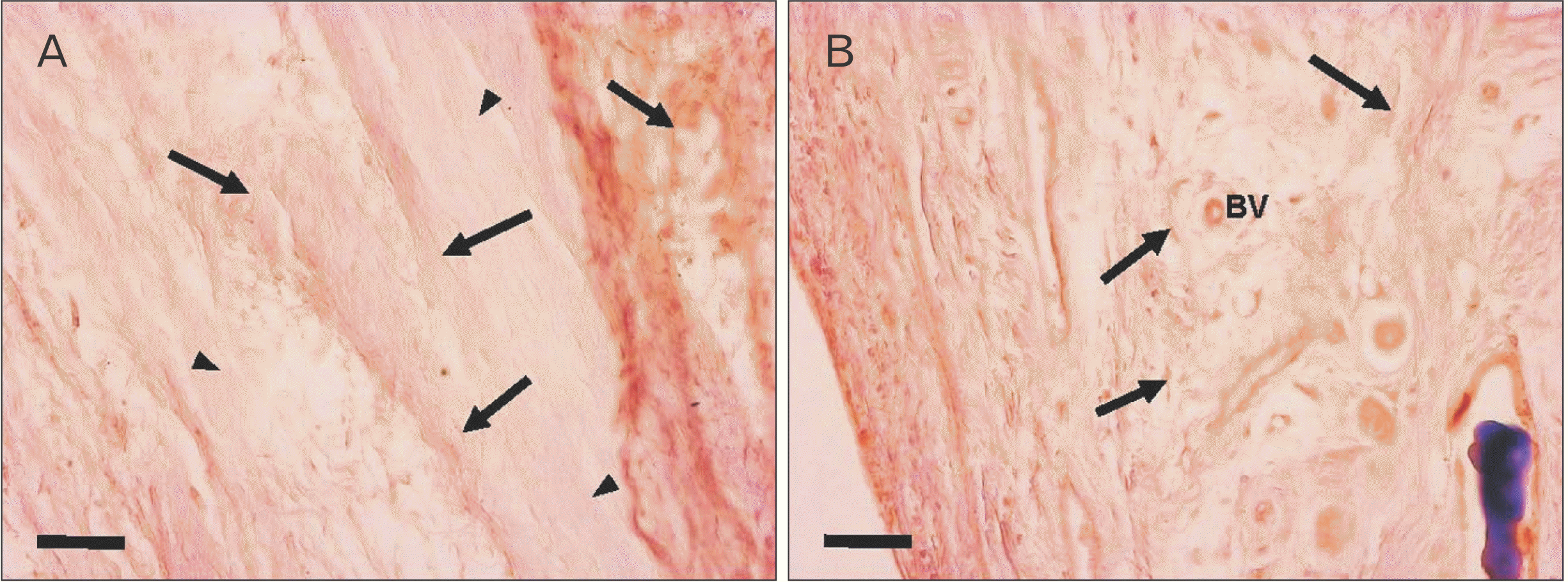
Fig. 8
Immunohistochemical staining image of fibrillin 1 in the fiber group of the thyroepiglottic ligament (×20). Samples from (A) corpses capable of oral intake, and (B) corpses who relied on enteral nutrition. Red arrows indicate fibrillin 1 antibody-positive microfibrils, which are presumed to be oxytalan fibers. Scale bar: 100 μm.
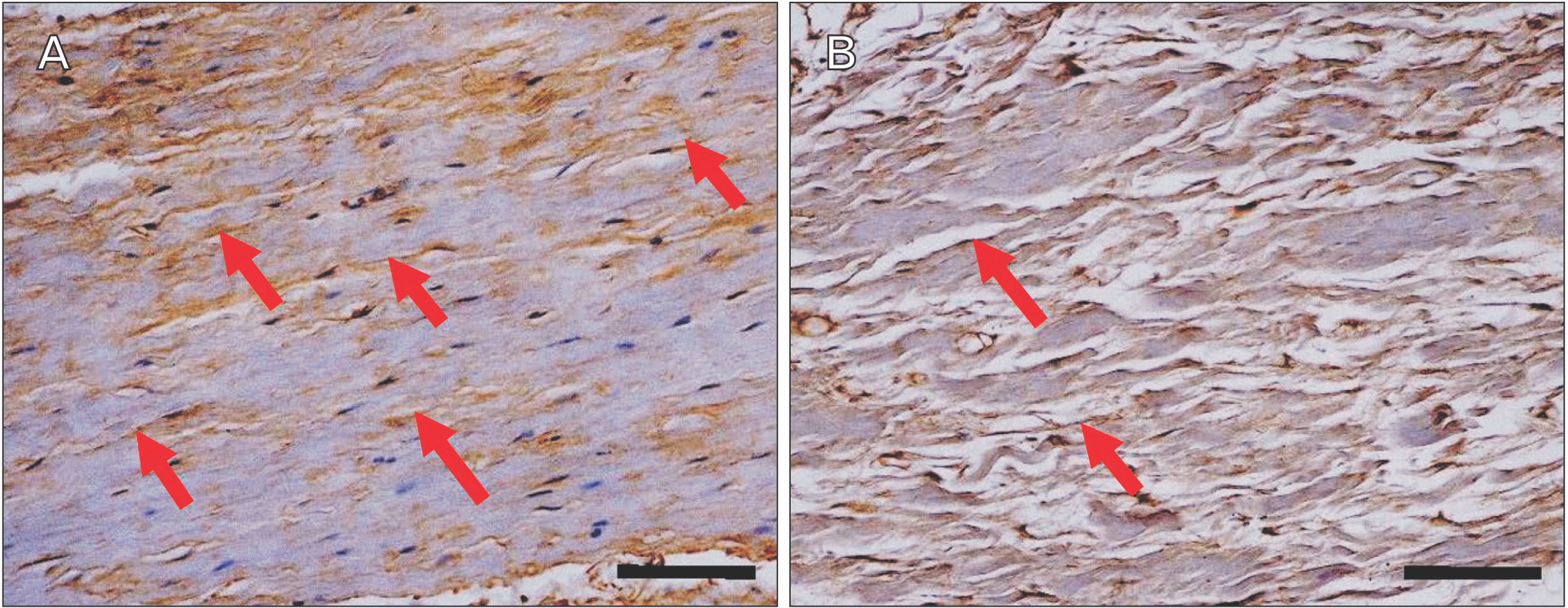
Fig. 9
Immunohistochemical staining image of fibrillin 1 in the fiber group of the hyoid–epiglottic ligament (×20). Samples from (A) corpses capable of oral intake, and (B) corpses who relied on enteral nutrition. Red arrows indicate fibrillin 1 antibody-positive microfibrils, which are presumed to be oxytalan fibers. Scale bar: 100 μm.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download