Abstract
The aim of this study was to elucidate the intramuscular arborization of the teres minor muslce for effective botulinum neurotoxin injection. Twelve specimens from 6 adult Korean cadavers (3 males and 3 females, age ranging from 66 to 78 years) were used in the study. The reference line between the 2/3 point of the axillary border of the scapula (0/5), where the muscle originates ant the insertion point of the greater tubercle of the humerus (5/5). The most intramuscular neural distribution was located on 1/5–3/5 of the muscle. The tendinous portion was observed in the 3/5–5/5. The result suggests the botulinum neurotoxin should be delivered in the 1/5–3/5 area of the teres minor muscle.
Botulinum neurotoxin has become a popular treatment in recent years due to its proven safety and effectiveness in reducing muscle tone from aesthetics to rehabilitations in focal spasticity of the upper limb, leading to an improvement in active upper limb function [1-9]. Several clinical trials have included the shoulder muscles, and they have shown positive results in reducing shoulder spasticity and improving shoulder function [10-16]. In fact, an observational study of chronic post-stroke spasticity has also demonstrated that botulinum toxin injections can reduce pain and improve shoulder function [17]. However, despite these findings, no specific injection points have been suggested for the teres minor muscle so far.
To achieve maximum effectiveness, administering botulinum neurotoxin injections requires precise dosage and location. Studies have shown that injections near the neural arborized region, which has a high density of neuromuscular junctions, are especially effective [18-24]. However, it can be challenging to locate small nerves accurately through visual inspection. To overcome this issue, researchers have used a technique called Sihler staining, which highlights nerves while making muscle fibers transparent [25-34]. The objective of the current study is to utilize Sihler staining to better understand the distribution of intramuscular nerves in the teres minor muscle.
This study was conducted in compliance with the Act on Dissection and Preservation of Corpses of the Republic of Korea (Act number: 14885) and approved by the Institutional Cadaver Research Committee of the College of Medicine, the Catholic University of Korea (MC22SISI0056).
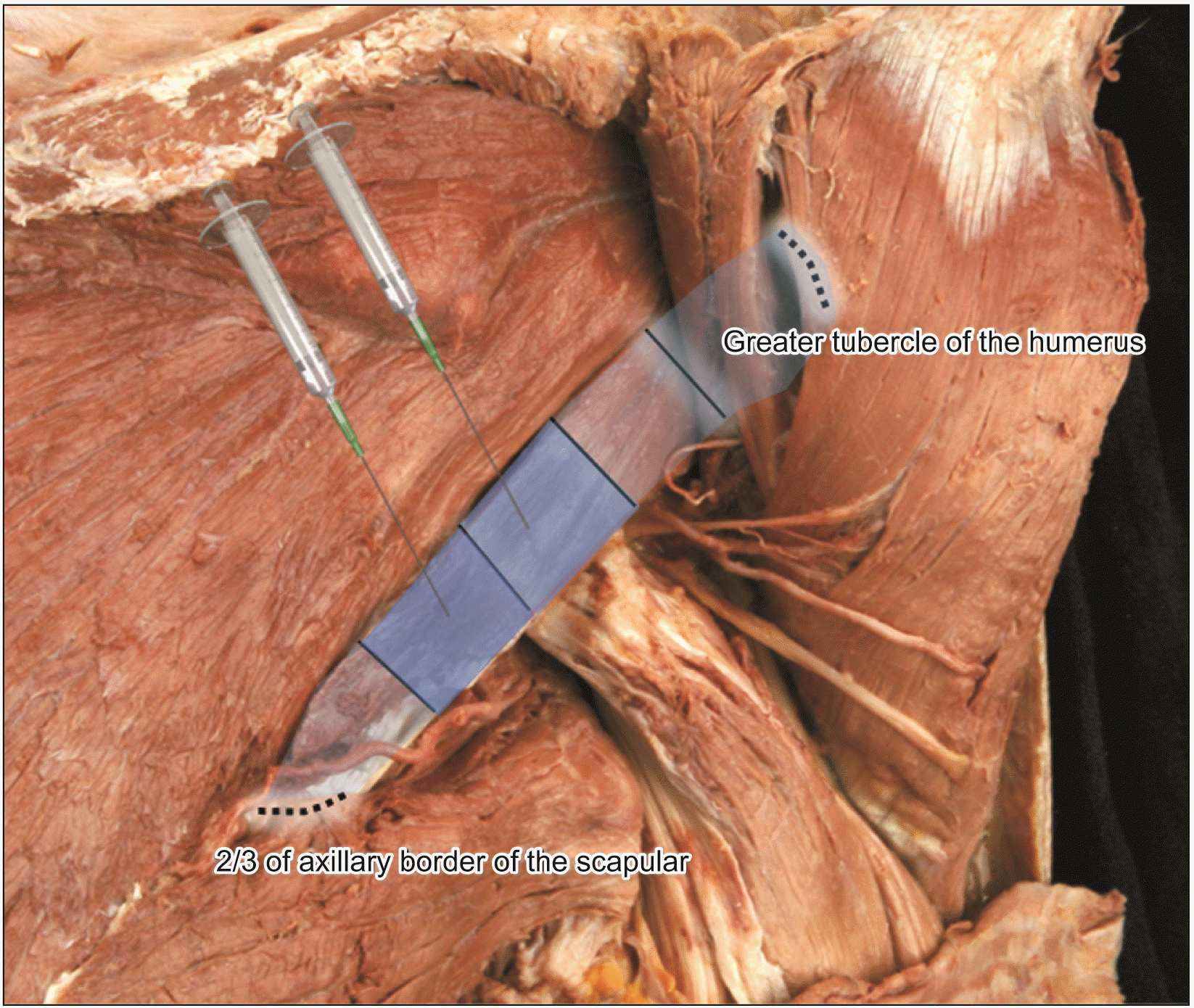
Before beginning the dissection, consent and approval were obtained from the families of the cadaver subjects. The study utilized a modified version of Sihler’s method to reveal the intramuscular nerve patterns in the teres minor muscle, using 6 cadavers. The cadavers were of Korean origin, 3 males and 3 females, age ranging from 66 to 78 years were used in the study. The twelve teres minor muscles were dissected and aligned according to anatomical structure (as depicted in Fig. 1) and stained to detect intramuscular neural distribution. The teres minor muscle was extracted from the 2/3 point of the axillary border of the scapula (0/5), where the muscle originates ant the insertion point of the greater tubercle of the humerus (5/5) (as illustrated in Fig. 2). The arborization patterns in the muscles were analyzed in relation to the vertical length of the muscle, which was divided into 5 parts. This division was based on previous studies that found botulinum neurotoxin to spread a few centimeters from the injection site [17-19].
The intramuscular neural arborization pattern of the teres minor muscles was studied using a modified Sihler’s staining technique, as shown in Figs. 3, 4. The muscle samples underwent multiple steps, which included fixation in non-neutral formalin for a month, treatment with potassium hydroxide and hydrogen peroxide to enhance transparency for a month, decalcification in a mixture of glycerin, acetic acid, and chloral hydrate for four weeks, staining with hematoxylin, glycerin, and chloral hydrate for five weeks, re-soaking in the decalcification solution to highlight the nerves, and finally display through immersion in glycerin solutions of increasing concentration (40%, 60%, 80%, and 100%). For a more comprehensive overview of the procedures, refer to Yi et al. [34].
Ten of the 12 specimens had a nerve entry point between the 2/3 point of the axillary border of the scapula (0/5), and greater tubercle of the humerus (5/5), while the other two specimen had a nerve entry point between 1/5 to 2/5.
The teres minor muscle originates from the back surface of the axillary border of the scapula, covering about two-thirds of its length. Additionally, it originates from two aponeurotic laminae that separate it from the infraspinatus and teres major muscles. The main function of the teres minor muscle is lateral rotation, and it also contributes to shoulder adduction in conjunction with the infraspinatus muscle. However, the two muscles are distinguished by their nerve supply, with the teres minor muscle being supplied by the axillary nerve and the infraspinatus muscle by the suprascapular nerve. If either muscle becomes stiff, it can restrict movement of the shoulder joint, leading to limitations in movement [35].
Chemical neurolysis with phenol, alcohol, and botulinum neurotoxin injections is a potential treatment for muscle stiffness [36]. Botulinum neurotoxin works by blocking the release of acetylcholine from nerve endings at the neuromuscular junction, resulting in selective effects on motor nerves without impacting sensory nerves [27, 37-39]. Compared to phenol and alcohol, botulinum neurotoxin has lower side effects, making it a popular option for managing spasticity. To achieve the maximum effect, it is recommended that these injections be administered close to areas where neuromuscular junctions are most densely distributed.
The muscles located at the back of the shoulder include the posterior deltoid, infraspinatus, and teres minor muscles, which can cause limited internal rotation due to muscle stiffness [40]. The findings of this study on the injection point may help to improve shoulder movement.
The significance of using neuromuscular junction-targeted botulinum neurotoxin injections has been confirmed in clinical studies in the biceps brachii muscle and iliopsoas muscle. Neuromuscular junction-oriented injections result in much greater volume reduction than control injections [18, 19].
In this study, the origin and insertion point of the teres minor muscle were selected as the reference point for injections, as they are easily identifiable, especially in patients with limited upper extremity movement that may make it challenging to locate other landmarks.
There are limited studies that have investigated the anatomy of the teres minor muscle. Our study identified the location of the intramuscular neural dense area of the nerve branch that connects to the teres minor muscle. This area was found to be located between 1/5 and 3/5 from the reference point and these areas should considered in clinical fields (Fig. 5). This finding could serve as a helpful guideline for injections targeting the teres minor muscle.
Acknowledgements
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind’s overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude.
Notes
References
1. Jacinto J, Camões-Barbosa A, Carda S, Hoad D, Wissel J. 2022; A practical guide to botulinum neurotoxin treatment of shoulder spasticity 1: anatomy, physiology, and goal setting. Front Neurol. 13:1004629. DOI: 10.3389/fneur.2022.1004629. PMID: 36324373. PMCID: PMC9618862. PMID: ffeba5c71fd142ffaeffa4fd6e8900b8.

2. Yi KH, Lee JH, Hu HW, Kim HJ. 2022; Anatomical proposal for botulinum neurotoxin injection for glabellar frown lines. Toxins (Basel). 14:268. DOI: 10.3390/toxins14040268. PMID: 35448877. PMCID: PMC9032255. PMID: dd131953c6e14366befdc986e94b15dd.

3. Yi KH, Lee HJ, Choi YJ, Lee K, Lee JH, Kim HJ. 2021; Anatomical guide for botulinum neurotoxin injection: application to cosmetic shoulder contouring, pain syndromes, and cervical dystonia. Clin Anat. 34:822–8. DOI: 10.1002/ca.23690. PMID: 32996645.

4. Yi KH, Lee JH, Lee K, Hu HW, Lee HJ, Kim HJ. 2022; Anatomical proposal for botulinum neurotoxin injection targeting the platysma muscle for treating platysmal band and jawline lifting: a review. Toxins (Basel). 14:868. DOI: 10.3390/toxins14120868. PMID: 36548765. PMCID: PMC9783622. PMID: 1fab78f1f2fb436e8f0adab9755ed1d2.

5. Yi KH, Lee JH, Kim GY, Yoon SW, Oh W, Kim HJ. 2022; Novel anatomical proposal for botulinum neurotoxin injection targeting lateral canthal rhytids. Toxins (Basel). 14:462. DOI: 10.3390/toxins14070462. PMID: 35878200. PMCID: PMC9316553. PMID: 46755210c7064a99b625f2d6b6dc6e0a.

6. Yi KH, Lee JH, Hu HW, Kim HJ. 2022; Novel anatomical guidelines on botulinum neurotoxin injection for wrinkles in the nose region. Toxins (Basel). 14:342. DOI: 10.3390/toxins14050342. PMID: 35622589. PMCID: PMC9144745. PMID: 95ea3c07c66d4527b5265c5ec719f652.

7. Yi KH, Lee HJ, Hur HW, Seo KK, Kim HJ. 2022; Guidelines for botulinum neurotoxin injection for facial contouring. Plast Reconstr Surg. 150:562e–71e. DOI: 10.1097/PRS.0000000000009444. PMID: 35759641.

8. Yi KH, Lee JH, Hu HW, Choi YJ, Lee K, Lee HJ, Kim HJ. 2023; Feb. 21. Novel anatomical proposal for botulinum neurotoxin injection targeting depressor anguli oris for treating drooping mouth corner. Anat Cell Biol. [Epub]. https://doi.org/10.5115/acb.22.258. DOI: 10.5115/acb.22.258. PMID: 36808109. PMCID: PMC10319486.

9. Yi KH, Lee JH, Hu HW, Park HJ, Bae H, Lee K, Kim HJ. 2023; Feb. 13. Novel anatomical guidelines for botulinum neurotoxin injection in the mentalis muscle: a review. Anat Cell Biol. [Epub]. https://doi.org/10.5115/acb.22.266. DOI: 10.5115/acb.22.266. PMID: 36796830.
10. Wissel J, Bensmail D, Scheschonka A, Flatau-Baqué B, Simon O, Althaus M, Simpson DM. 2020; Post hoc analysis of the improvement in shoulder spasticity and safety observed following treatment with incobotulinumtoxinA. J Rehabil Med. 52:jrm00028. DOI: 10.2340/16501977-2651. PMID: 32025741.
11. Lim JY, Koh JH, Paik NJ. 2008; Intramuscular botulinum toxin-A reduces hemiplegic shoulder pain: a randomized, double-blind, comparative study versus intraarticular triamcinolone acetonide. Stroke. 39:126–31. DOI: 10.1161/STROKEAHA.107.484048. PMID: 18048857.

12. Yelnik AP, Colle FM, Bonan IV, Vicaut E. 2007; Treatment of shoulder pain in spastic hemiplegia by reducing spasticity of the subscapular muscle: a randomised, double blind, placebo controlled study of botulinum toxin A. J Neurol Neurosurg Psychiatry. 78:845–8. DOI: 10.1136/jnnp.2006.103341. PMID: 17088333. PMCID: PMC2117719.

13. Marco E, Duarte E, Vila J, Tejero M, Guillen A, Boza R, Escalada F, Espadaler JM. 2007; Is botulinum toxin type A effective in the treatment of spastic shoulder pain in patients after stroke? A double-blind randomized clinical trial. J Rehabil Med. 39:440–7. DOI: 10.2340/16501977-0066. PMID: 17624477.

14. Turner-Stokes L, Fheodoroff K, Jacinto J, Maisonobe P. 2013; Results from the Upper Limb International Spasticity Study-II (ULISII):a large, international, prospective cohort study investigating practice and goal attainment following treatment with botulinum toxin A in real-life clinical management. BMJ Open. 3:e002771. DOI: 10.1136/bmjopen-2013-002771. PMID: 23794582. PMCID: PMC3686177.
15. Wissel J, Bensmail D, Ferreira JJ, Molteni F, Satkunam L, Moraleda S, Rekand T, McGuire J, Scheschonka A, Flatau-Baqué B, Simon O, Rochford ET, Dressler D, Simpson DM. 2017; Safety and efficacy of incobotulinumtoxinA doses up to 800 U in limb spasticity: the TOWER study. Neurology. 88:1321–8. DOI: 10.1212/WNL.0000000000003789. PMID: 28283596. PMCID: PMC5379931.

16. Gracies JM, Brashear A, Jech R, McAllister P, Banach M, Valkovic P, Walker H, Marciniak C, Deltombe T, Skoromets A, Khatkova S, Edgley S, Gul F, Catus F, De Fer BB, Vilain C, Picaut P. 2015; Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double-blind randomised controlled trial. Lancet Neurol. 14:992–1001. DOI: 10.1016/S1474-4422(15)00216-1. PMID: 26318836.

17. Khan P, Riberto M, Frances JA, Chueire R, Amorim ACFG, Xerez D, Chung TM, Mercuri LHC, Longo AL, Lianza S, Maisonobe P, Ruiz-Schutz VC. 2020; The effectiveness of botulinum toxin type A (BoNT-A) treatment in Brazilian patients with chronic post-stroke spasticity: results from the observational, multicenter, prospective BCause study. Toxins (Basel). 12:770. DOI: 10.3390/toxins12120770. PMID: 33291807. PMCID: PMC7762077. PMID: f655308c31804bc78bdfd0a1c059f2d1.

18. Gracies JM, Lugassy M, Weisz DJ, Vecchio M, Flanagan S, Simpson DM. 2009; Botulinum toxin dilution and endplate targeting in spasticity: a double-blind controlled study. Arch Phys Med Rehabil. 90:9–16.e2. DOI: 10.1016/j.apmr.2008.04.030. PMID: 19154823.
19. Van Campenhout A, Verhaegen A, Pans S, Molenaers G. 2013; Botulinum toxin type A injections in the psoas muscle of children with cerebral palsy: muscle atrophy after motor end plate-targeted injections. Res Dev Disabil. 34:1052–8. DOI: 10.1016/j.ridd.2012.11.016. PMID: 23295965.
20. Yi KH, Lee HJ, Seo KK, Kim HJ. 2022; Botulinum neurotoxin injection guidelines regarding flap surgeries in breast reconstruction. J Plast Reconstr Aesthet Surg. 75:503–5. DOI: 10.1016/j.bjps.2021.09.081. PMID: 34776389.

21. Yi KH, Lee HJ, Lee JH, Seo KK, Kim HJ. 2021; Application of botulinum neurotoxin injections in TRAM flap for breast reconstruction: intramuscular neural arborization of the rectus abdominis muscle. Toxins (Basel). 13:269. DOI: 10.3390/toxins13040269. PMID: 33918558. PMCID: PMC8070362. PMID: 15169c322e5c4f158142057c4d95936b.

22. Yi KH, Lee HJ, Lee JH, Lee KL, Kim HJ. 2021; Effective botulinum neurotoxin injection in treating iliopsoas spasticity. Clin Anat. 34:431–6. DOI: 10.1002/ca.23670. PMID: 32805076.

23. Yi KH, Choi YJ, Cong L, Lee KL, Hu KS, Kim HJ. 2020; Effective botulinum toxin injection guide for treatment of cervical dystonia. Clin Anat. 33:192–8. DOI: 10.1002/ca.23430. PMID: 31301235.

24. Yi KH, Rha DW, Kim HJ, Hu KS. 2016; Reply: optimizing efficacy of botulinum toxin injections using ultrasound guidance. Muscle Nerve. 54:513–4. DOI: 10.1002/mus.25213. PMID: 27287828.
25. Lee JH, Lee BN, An X, Chung RH, Han SH. 2011; Location of the motor entry point and intramuscular motor point of the tibialis posterior muscle: for effective motor point block. Clin Anat. 24:91–6. DOI: 10.1002/ca.21062. PMID: 21154644.

26. Oddy MJ, Brown C, Mistry R, Eastwood DM. 2006; Botulinum toxin injection site localization for the tibialis posterior muscle. J Pediatr Orthop B. 15:414–7. DOI: 10.1097/01.bpb.0000228387.94065.ff. PMID: 17001247.

27. Rha DW, Yi KH, Park ES, Park C, Kim HJ. 2016; Intramuscular nerve distribution of the hamstring muscles: application to treating spasticity. Clin Anat. 29:746–51. DOI: 10.1002/ca.22735. PMID: 27213466.

28. Yi KH, Rha DW, Lee SC, Cong L, Lee HJ, Lee YW, Kim HJ, Hu KS. 2016; Intramuscular nerve distribution pattern of ankle invertor muscles in human cadaver using Sihler stain. Muscle Nerve. 53:742–7. DOI: 10.1002/mus.24939. PMID: 26467315.

29. Yi KH, Lee KL, Lee JH, Hu HW, Lee K, Seo KK, Kim HJ. 2021; Guidelines for botulinum neurotoxin injections in piriformis syndrome. Clin Anat. 34:1028–34. DOI: 10.1002/ca.23711. PMID: 33347678.

30. Yi KH, Lee KL, Lee JH, Hu HW, Kim HJ. 2022; Guidance to trigger point injection for treating myofascial pain syndrome: intramuscular neural distribution of the quadratus lumborum. Clin Anat. 35:1100–6. DOI: 10.1002/ca.23918. PMID: 35655442.

31. Yi KH, Lee JH, Lee DK, Hu HW, Seo KK, Kim HJ. 2021; Anatomical locations of the motor endplates of sartorius muscle for botulinum toxin injections in treatment of muscle spasticity. Surg Radiol Anat. 43:2025–30. DOI: 10.1007/s00276-021-02813-7. PMID: 34378107. PMCID: PMC8354843.

32. Yi KH, Lee JH, Kim HM, Kim HJ. 2022; The botulinum neurotoxin for pain control after breast reconstruction: neural distribution of the pectoralis major muscle. Reg Anesth Pain Med. 47:322–6. DOI: 10.1136/rapm-2021-102653. PMID: 35039438.

33. Yi KH, Lee JH, Kim HJ. 2022; Intramuscular neural distribution of the serratus anterior muscle: regarding botulinum neurotoxin injection for treating myofascial pain syndrome. Toxins (Basel). 14:271. DOI: 10.3390/toxins14040271. PMID: 35448880. PMCID: PMC9033065. PMID: c4ef8fa6e12442a990c0be9185a526e5.

34. Yi KH, Lee JH, Hur HW, Lee HJ, Choi YJ, Kim HJ. 2023; Jan. 6. Distribution of the intramuscular innervation of the triceps brachii: clinical importance in the treatment of spasticity with botulinum neurotoxin. Clin Anat. [Epub]. https://doi.org/10.1002/ca.24004. DOI: 10.1002/ca.24004. PMID: 36606364.

35. Hung CJ, Hsieh CL, Yang PL, Lin JJ. 2010; Relationships between posterior shoulder muscle stiffness and rotation in patients with stiff shoulder. J Rehabil Med. 42:216–20. DOI: 10.2340/16501977-0504. PMID: 20411215.
36. Deltombe T, De Wispelaere JF, Gustin T, Jamart J, Hanson P. 2004; Selective blocks of the motor nerve branches to the soleus and tibialis posterior muscles in the management of the spastic equinovarus foot. Arch Phys Med Rehabil. 85:54–8. DOI: 10.1016/S0003-9993(03)00405-2. PMID: 14970968.
37. Bhakta BB, Cozens JA, Bamford JM, Chamberlain MA. 1996; Use of botulinum toxin in stroke patients with severe upper limb spasticity. J Neurol Neurosurg Psychiatry. 61:30–5. DOI: 10.1136/jnnp.61.1.30. PMID: 8676154. PMCID: PMC486452.

38. Yi KH, Lee HJ, Seo KK, Kim HJ. 2022; Intramuscular neural arborization of the latissimus dorsi muscle: application of botulinum neurotoxin injection in flap reconstruction. Toxins (Basel). 14:107. DOI: 10.3390/toxins14020107. PMID: 35202134. PMCID: PMC8878018. PMID: 0c7534ebd76b4902bff96b4bfe81c6b3.

39. Yi KH, Lee HJ, Choi YJ, Lee JH, Hu KS, Kim HJ. 2020; Intramuscular neural distribution of rhomboid muscles: evaluation for botulinum toxin injection using modified Sihler's method. Toxins (Basel). 12:289. DOI: 10.3390/toxins12050289. PMID: 32375284. PMCID: PMC7291336. PMID: 2a43e00b63b34292bd34bcaa4ce50d09.

40. Lin JJ, Yang JL. 2006; Reliability and validity of shoulder tightness measurement in patients with stiff shoulders. Man Ther. 11:146–52. DOI: 10.1016/j.math.2005.05.002. PMID: 16095946.

Fig. 1
The teres minor muscle has been revealed after removing posterior deltoid and dissected with surrounding muscles of infraspinatus, teres major, and long head of triceps brachii.
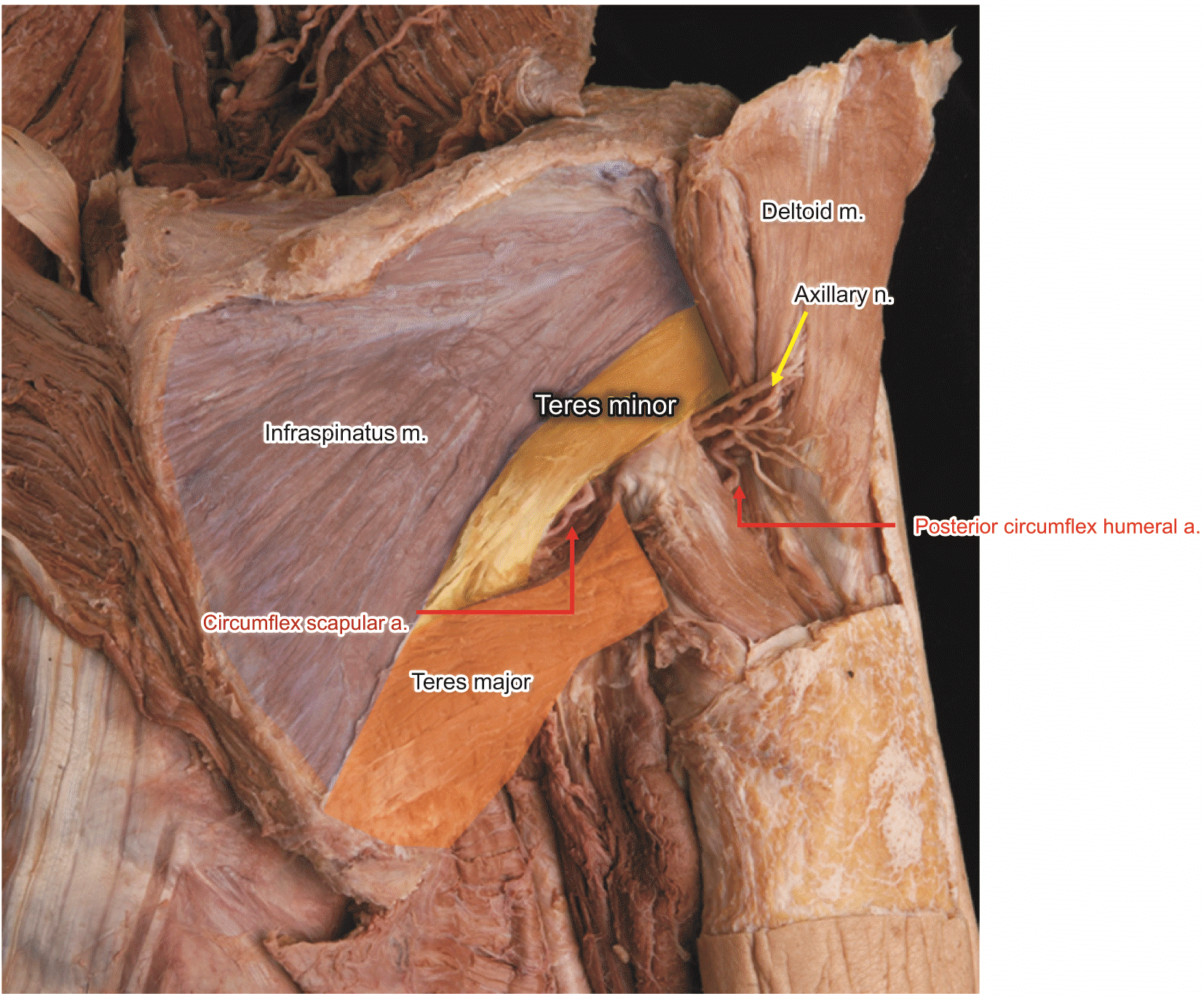
Fig. 2
The teres minor muscle was harvested from the 2/3 point of the axillary border of the scapula (0/5), where the muscle originates, and the insertion point of the greater tubercle of the humerus (5/5). The arborization patterns in the muscles were elucidated with respect to the horizontal length of the muscle with 5 divisions.
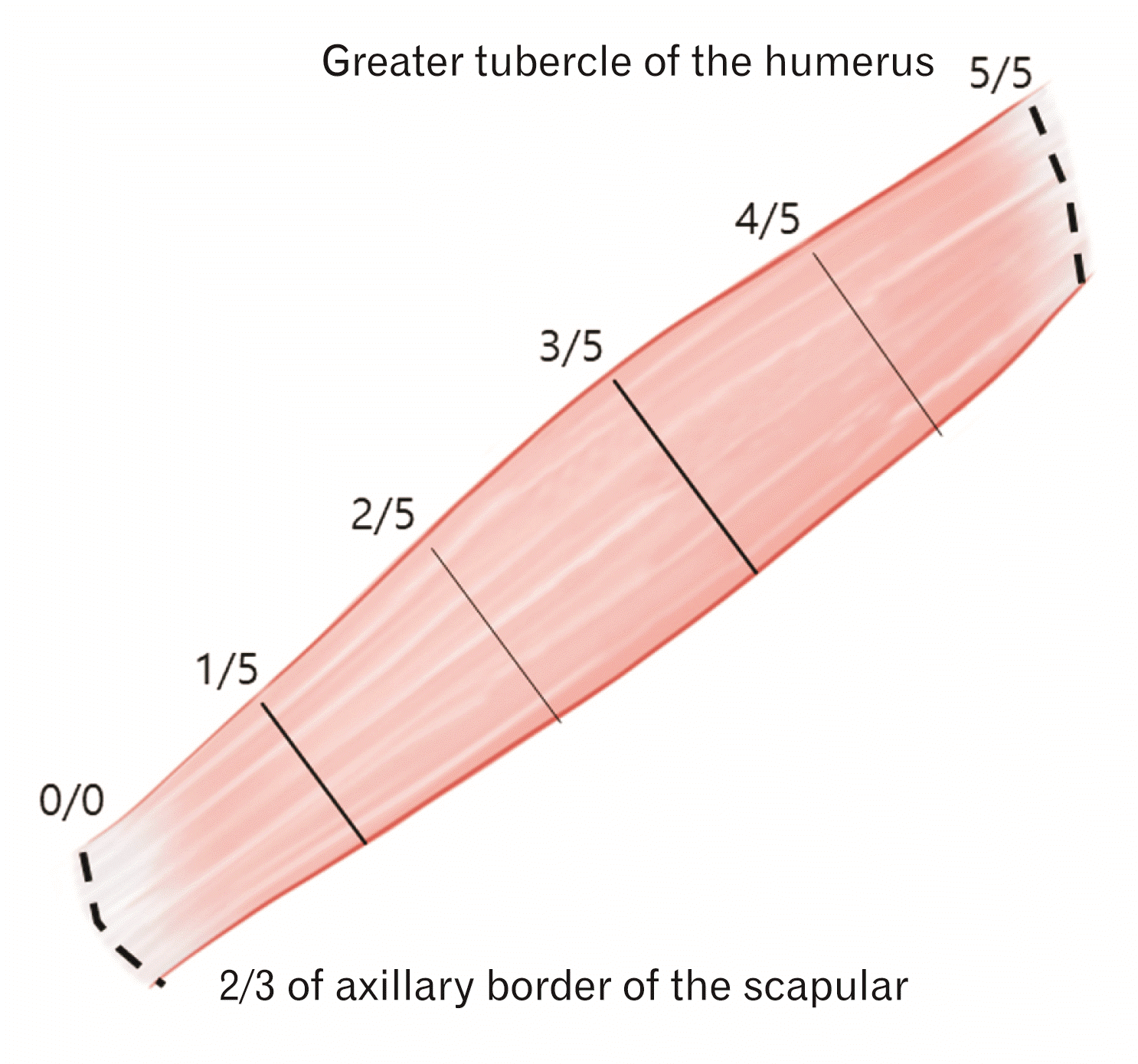
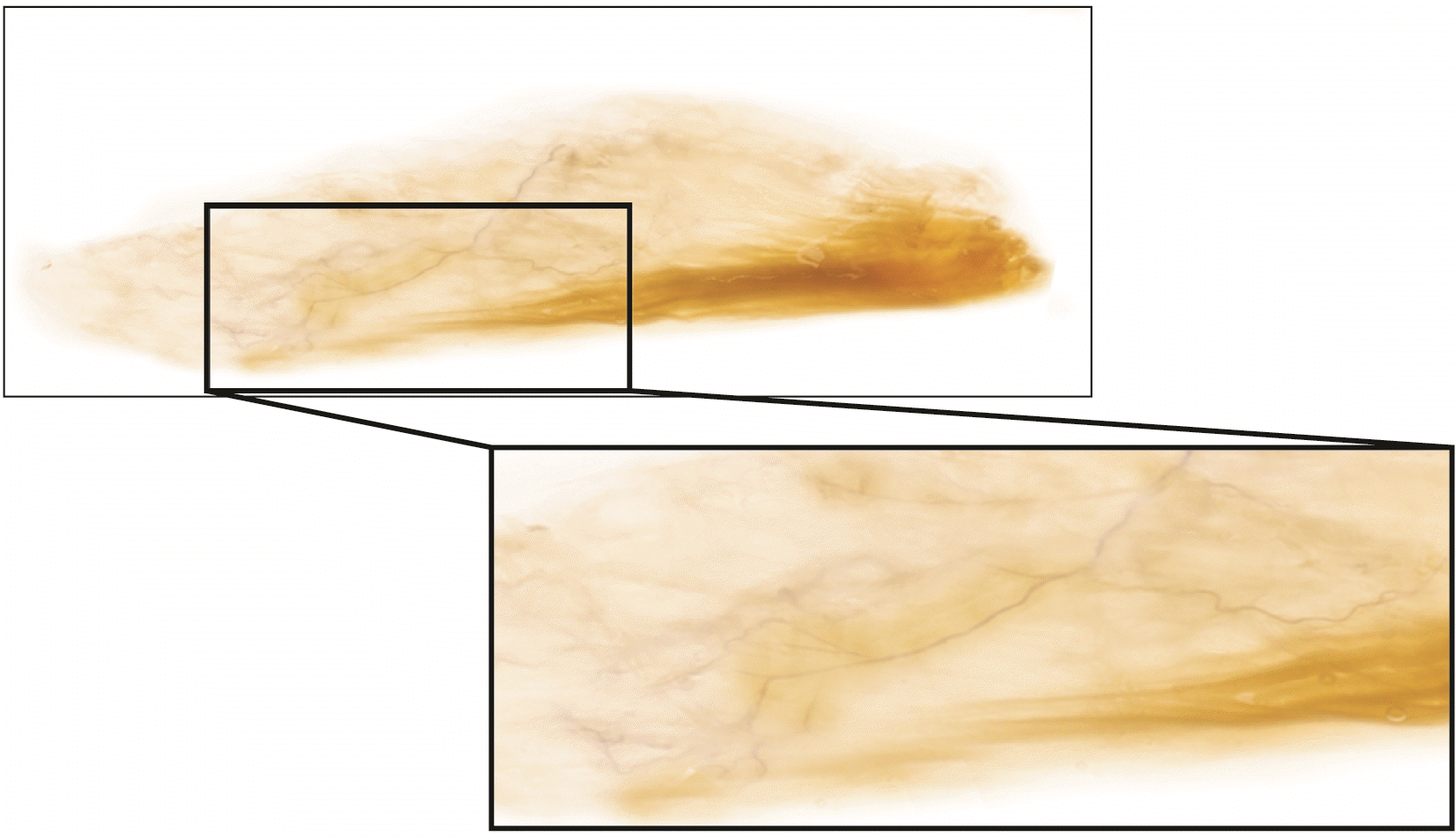
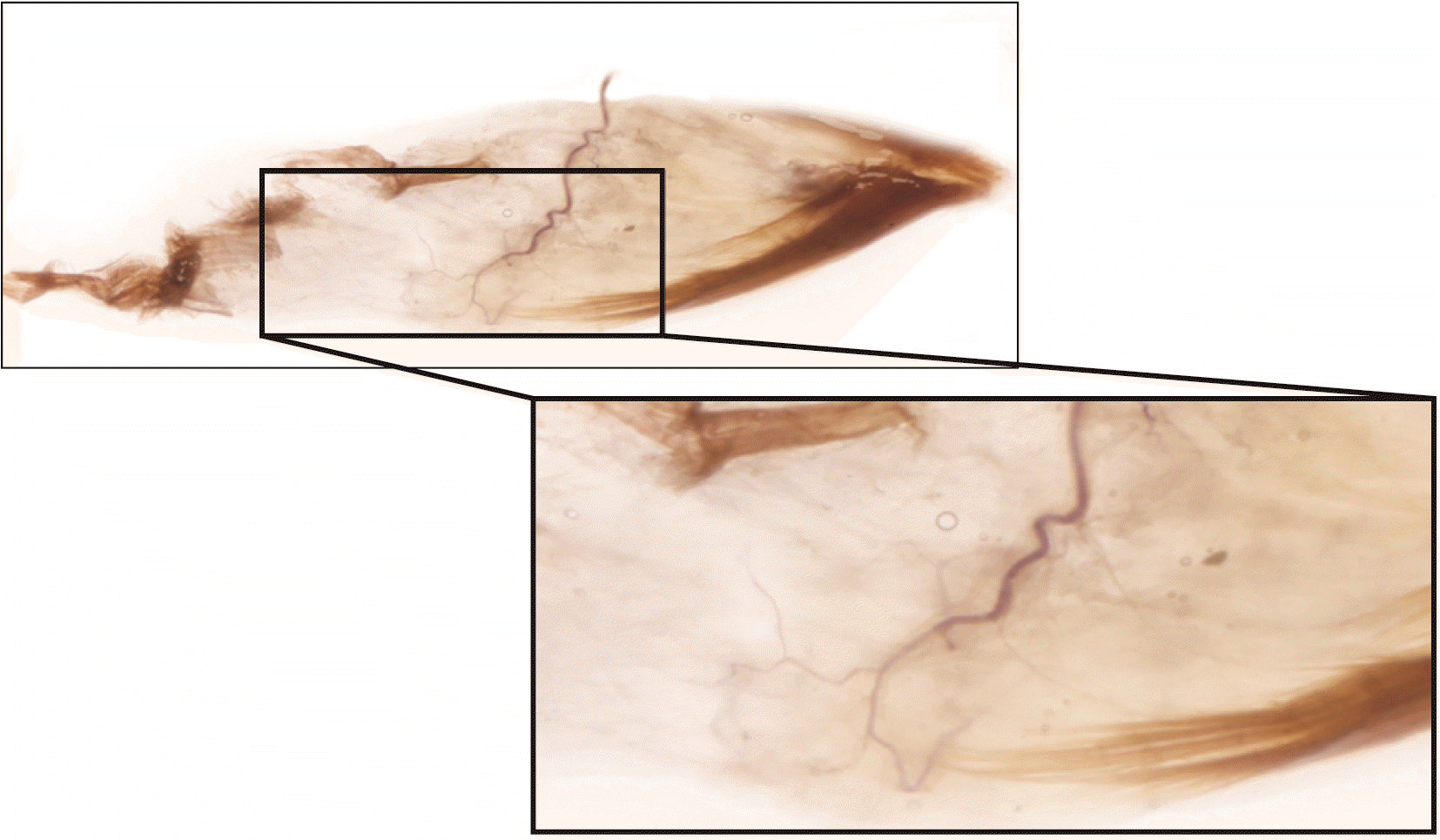
Fig. 3
A specimen of Sihler’s stained teres minor muscle with enlarged panels of intramuscular arborizations. The intramuscular pattern and nerve entry points are revealed by dissection and staining.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download