Abstract
The laryngopharyngeal nerve has received much less attention that the other contributions to the pharyngeal plexus i.e., glossopharyngeal and vagus nerves. Often, in descriptions and depictions, the nerve is simply labeled as the sympathetic contribution to the pharyngeal plexus. As there is such scant information available regarding this nerve, the present review was performed. Very little is found in the extant medical literature regarding the laryngopharyngeal nerve. However, based on available data, the nerve is a consistent contributory to the pharyngeal plexus and serves other adjacent areas e.g., carotid body. Therefore, a better understanding of this structure’s anatomy is important for those who operate in this area. Further studies are necessary to better elucidate the true function of the laryngopharyngeal nerve.
The earliest reference to the laryngopharyngeal nerve (Fig. 1) dates back to ancient Rome. Circa 192 of the Common Era, Galen described contributing branches of the vagus nerve (X cranial nerve), which would later come to be known as the laryngopharyngeal branches and actually be understood to arise from the superior cervical ganglia [1]. In 1664 Willis [2] elaborated on ramifications of laryngopharyngeal branches of the superior cervical ganglion, localizing terminal innervations to the sphincter of the throat, though no nomenclature or illustration was provided. Soon thereafter in 1684, Vieussens [3] described medial branches of the superior cervical ganglia, which supplied the carotid body and passed to the side of the pharynx. The anatomical description was later expanded to include connecting branches of the glossopharyngeal (IX cranial) nerve, eventually forming the pharyngeal plexus [4, 5]. Reports in the literature demonstrate that, over time, the laryngopharyngeal nerve has had a number of synonyms including the laryngeal branch of the cervical intercostal trunk, the superior soft nerve, and the soft branch of the superior cervical ganglion [5, 6].
The superior cervical ganglia derive from neural crest cells, which come from the ectoderm in embryological development [7, 8]. The superior cervical ganglion is the largest of the cervical ganglia and consists of the fused ganglia of C1 to C4. It is involved explicitly with sympathetic efferent innervation, particularly to the face and head [9]. It is the largest of the three ganglia of the cervical sympathetic trunk, the other two being the stellate and middle cervical ganglion [10]. It is situated at the level of the second and third cervical vertebrae, anterior to the longus capitis muscle and posterior to the internal carotid artery and its carotid sheath [10]. It is connected to the middle cervical ganglion inferiorly by the sympathetic trunk. It gives rise to lateral, medial and anterior branches. The medial branches of the superior cervical ganglion consist of laryngopharyngeal and cardiac branches [11]. The laryngopharyngeal branches (Fig. 2) supply the carotid body and travel to the side of the pharynx, to form the pharyngeal plexus with the glossopharyngeal (IX cranial nerve) and the vagus nerve rami.
Branches from the ascending pharyngeal artery, coming off of the external carotid artery, provide blood supply to the superior cervical ganglia and its branches such as the laryngopharyngeal nerve [9]. Venous drainage is via the internal jugular vein through direct posterior branches. There is a noted deficiency in gross blood supply at the junction of the upper one third and lower two-thirds of the neck; this is important for surgical considerations during procedures that involve anterior neck dissections, such as anterior cervical discectomy and fusion [12].
In general, the superior cervical ganglia provide the postsynaptic sympathetic fibers to the intracranial structures [13].Additionally, the superior cervical ganglia supply gray rami communicants to the ventral rami of components of the cervical plexus (Fig. 2) [14]. The superior cervical ganglia connect to the next closest ganglia, the middle cervical ganglia, inferiorly via the sympathetic trunk [15].
The pharyngeal plexus (Fig. 3) has a pivotal role in swallowing, as it innervates all the muscles of the pharynx, with the exception of the stylopharyngeus, which is directly innervated by the glossopharyngeal nerve [16]. The first mixed trunk of the pharyngeal plexus innervates the central part of the superior pharyngeal constrictor, then the salpingo- and palatopharyngeus muscles. Caudally, the plexus innervates the middle and inferior pharyngeal constrictors. The intrinsic muscles of the larynx are innervated by the recurrent laryngeal nerve, which is not known to have any contribution from the laryngopharyngeal branches of the superior cervical ganglion. The cricothyroid muscle of the larynx is innervated by the external laryngeal branch of the superior laryngeal nerve of the vagus nerve. The laryngopharyngeal branches which supply the carotid body provide afferent signals to the central nervous system based on the partial pressure of oxygen in the bloodstream [17]. Cell bodies of these sensory afferent nerve terminals lie in the petrosal ganglion.
When the superior cervical ganglion or the sympathetic fibers are damaged, Horner’s syndrome can develop. Damage to the nerve fibers can be caused by tension, for instance, retracting the longus colli muscle or carotid sheath, indirectly tensioning the sympathetic trunk during anterior neck surgeries such as anterior cervical discectomy and fusions [9, 18]. Such procedures, including anterior cervical discectomies, can cause a disruption of the blood supply to the superior cervical ganglia resulting in ischemic damage to the ganglia. This insult can also cause Horner-like symptoms such as ptosis, miosis, enophthalmos, facial flushing, and anhidrosis [8]. In addition to decreased sympathetic innervation, dysfunction of fibers associated with the laryngopharyngeal nerve have important clinical relevance. Tension or damaged to the external carotid plexus can cause tension on the sympathetic trunk, causing Harlequin syndrome which frequently accompanies Horner’s syndrome. Similarly, the plexus surrounding the external carotid artery, and their branches, could participated in the pathophysiological process of Sluder’s neuralgia or pterygopalatine neuralgia [18]. Moreover, function of the fast-outer layer of the pharyngeal muscles is diminished in patients with idiopathic Parkinson’s disease and cervical ganglioneuromas in the pediatric population have both been reported to cause dysphagia [19, 20].
Hara et al. [21] have studied the superior cervical ganglion in 10 human fetuses. Interestingly, they found that the laryngopharyngeal nerve variably arises solely from the external carotid plexus, the superior cervical ganglion, or both. When the laryngopharyngeal nerve arises only from the superior cervical ganglion, several branches are found near the origin of the external carotid plexus. In all cases, the nerve sends branches that join the superior laryngeal nerve, external carotid plexus, and pharyngeal plexus [21].
In one study, there was anatomic variation in origin of the laryngopharyngeal branch in the dromedary camel [22]. They originated from the following locations: (1) ventral pole of the superior cervical ganglion, (2) the medial surface of the proximal sympathetic trunk shortly after entrance into the superior cervical ganglion, or (3) the medial aspect of the rostral half of the superior cervical ganglion near the external carotid nerve in a common trunk with the carotid sinus branch, which has been described variable as arising from cranial nerve IX alone, from both cranial nerves IX and X, or having a branch derived from the sympathetic trunk.
There have been only several mentions of the laryngopharyngeal nerve in animal studies within the literature, aside from that in the dromedary camel [23, 24]. However, mention of a branch of the superior cervical ganglia and communicating with the cranial laryngeal nerve in several papers may be a direct reference to the laryngopharyngeal nerve, that nomenclature is not utilized [25, 26].
Additionally, studies in birds have suggested that a distinct laryngopharyngeal nerve innervates laryngeal and pharyngeal muscles along with the glands of the larynx and trachea. Avian models support the role of the laryngopharyngeal nerve as an essential part of the sympathetic nervous system [27]. Studies in crocodiles suggest a similar course to that in birds, with the laryngopharyngeal nerve emerging from the petrosal ganglion and carrying autonomic fibers to target structures [27, 28].
The laryngopharyngeal nerve has historically received less attention than the, for example, vagus nerve branches to the pharyngeal plexus (Fig. 4). Although its exact function for this intricate plexus of nerve fibers of the pharynx is not completely understood, these fibers should not be overlooked during procedures in the area that might result in iatrogenic damage of these fibers or near fibers, causing mainly temporary or permanent Horner’s syndrome, and other neurological disorders such as Harlequin syndrome and Sluder’s neuralgia. Further studies aimed at the anatomy and physiology of the laryngopharyngeal nerve are warranted.
Acknowledgements
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind’s overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude. The authors state that every effort was made to follow all local and international ethical guidelines and laws that pertain to the use of human cadaveric donors in anatomical research [29, 30].
Notes
Author Contributions
Conceptualization: SS, RST, JI. Data acquisition: SS, ALP. Data analysis or interpretation: RST, JI, ASD. Drafting of the manuscript: SS, ALP. Critical revision of the manuscript: JJC, AC (Arada Chaiyamoon), FR, AC (Ana Carrera). Approval of the final version of the manuscript: all authors.
References
1. Tibbetts PE. 2015; Neuroanatomical terminology: a lexicon of classical origins and historical foundations by Larry W. Swanson. Q Rev Biol. 90:223–4. DOI: 10.1086/681483.
2. Willis T. 1664. Cerebri anatome: cui accessit nervorum descriptio et usus]. Typis Tho. Roycroft, Impensis Jo. Martyn & Ja. Allestry;London:
3. Vieussens R. 1684. Neurographia universalis. Apud Joannem Certe Lugduni.
4. Huber JJ. 1744. Epistola anatomica de nervo intercostali de nervis octavi et noni paris deqve accessorio; nonnvlla tradens ad virvm ilustrem D.D. Wolrath wigand potentissimi svecorvm regis consiliarivm et archiatrvm ioh. Iacobi huberi D. Vandenhoeck;Göttingen:
5. Neubauer JE. 1772. Descriptio anatomica nervorum cardiacorum. In Officina Fleischeriana;DOI: 10.1163/1574-9347_dnp_e315900.
6. Lobstein JF. 1823. De nervi sympathetici humani fabrica, usu et morbis, commentatio anatomico-physiologico-pathologica. Levrault.
7. Moriyama H, Shimada K, Goto N. 1995; Morphometric analysis of neurons in ganglia: geniculate, submandibular, cervical spinal and superior cervical. Okajimas Folia Anat Jpn. 72:185–90. DOI: 10.2535/ofaj1936.72.4_185. PMID: 8570139.

8. Kameda Y, Saitoh T, Nemoto N, Katoh T, Iseki S. 2012; Hes1 is required for the development of the superior cervical ganglion of sympathetic trunk and the carotid body. Dev Dyn. 241:1289–300. DOI: 10.1002/dvdy.23819. PMID: 22689348.

9. Maningat AL, Munakomi S. 2022. Neuroanatomy, superior cervical ganglion. StatPearls. StatPearls Publishing;DOI: 10.53347/rid-98669.
10. Yokota H, Mukai H, Hattori S, Yamada K, Anzai Y, Uno T. 2018; MR imaging of the superior cervical ganglion and inferior ganglion of the vagus nerve: structures that can mimic pathologic retropharyngeal lymph nodes. AJNR Am J Neuroradiol. 39:170–6. DOI: 10.3174/ajnr.A5434. PMID: 29122764. PMCID: PMC7410715.

11. Janfaza P. 2001. Surgical anatomy of the head and neck. Lippincott Williams & Wilkins;DOI: 10.1001/archotol.1938.00650030527016.
12. Tubbs RS, Salter G, Wellons JC 3rd, Oakes WJ. 2002; Blood supply of the human cervical sympathetic chain and ganglia. Eur J Morphol. 40:283–8. DOI: 10.1076/ejom.40.5.283.28905. PMID: 15101443.

13. Goosmann MM, Dalvin M. 2022. Anatomy, head and neck, deep petrosal nerve. StatPearls. StatPearls Publishing;DOI: 10.53347/rid-56609.
14. Waxenbaum JA, Reddy V, Bordoni B. 2022. Anatomy, head and neck, cervical nerves. StatPearls. StatPearls Publishing.
15. Fazliogullari Z, Kilic C, Karabulut AK, Yazar F. 2016; A morphometric analysis of the superior cervical ganglion and its surrounding structures. Surg Radiol Anat. 38:299–302. DOI: 10.1007/s00276-015-1551-3. PMID: 26364034.

16. Gutierrez S, Iwanaga J, Pekala P, Yilmaz E, Clifton WE, Dumont AS, Tubbs RS. 2021; The pharyngeal plexus: an anatomical review for better understanding postoperative dysphagia. Neurosurg Rev. 44:763–72. DOI: 10.1007/s10143-020-01303-5. PMID: 32318923.

17. Nurse CA, Piskuric NA. 2013; Signal processing at mammalian carotid body chemoreceptors. Semin Cell Dev Biol. 24:22–30. DOI: 10.1016/j.semcdb.2012.09.006. PMID: 23022231.

18. Razipour SE, Zarrintan S, Mathkour M, Iwanaga J, Dumont AS, Tubbs RS. 2021; Review of the external carotid plexus: anatomy, function, and clinical manifestations. Anat Cell Biol. 54:137–42. DOI: 10.5115/acb.20.308. PMID: 33731490. PMCID: PMC8225485.

19. Mu L, Sanders I. 2007; Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol. 116:604–17. DOI: 10.1177/000348940711600809. PMID: 17847729.

20. Lima AF, Moreira FC, Menezes A, Dias L. 2018; Cervical ganglioneuroma in pediatric age: a case report. Turk Arch Otorhinolaryngol. 56:237–40. DOI: 10.5152/tao.2018.3690. PMID: 30701121. PMCID: PMC6340319. PMID: 170080a138274bfaa143b7d7c47ce9d6.

21. Hara I, Tanuma K, Suzuki K. 1993; Morphology of the ganglion cervicale superius in human fetuses and an adult. Kaibogaku Zasshi. 68:544–63. Japanese.
22. Nourinezhad J, Mazaheri Y, Biglari Z. 2015; Detailed anatomy of the cranial cervical ganglion in the dromedary camel (Camelus dromedarius). Anat Rec (Hoboken). 298:1479–91. DOI: 10.1002/ar.23169. PMID: 25950508.
23. Sisson S, Grossman JD, Getty R. 1975. Sisson and Grossman's the anatomy of the domestic animals. 5th ed. Saunders.
24. Ari HH, Soyguder Z, Cinaroglu S. 2010; Macroanatomy of the cranial cervical ganglion in Angora goats. Vet Med. 55:389–93. DOI: 10.17221/2952-VETMED. PMID: 8fec90b10ede40adaab6ecfaaa7245cb.

25. Hedger JH, Webber RH. 1976; Anatomical study of the cervical sympathetic trunk and ganglia in the albino rat (Mus norvegicus albinus). Acta Anat (Basel). 96:206–17. DOI: 10.1159/000144674. PMID: 970104.
26. Kabak M. 2007; The gross anatomy of the cranial cervical ganglion in the guinea pig (Cavia porcellus). Vet Res Commun. 31:1–7. DOI: 10.1007/s11259-006-3398-x. PMID: 17186407.

27. Nieuwenhuys R, Donkelaar HJ, Nicholson C. 1997. The central nervous system of vertebrates. Springer;DOI: 10.1007/978-3-642-18262-4.
28. Gaskell WH. 1886; On the structure, distribution and function of the nerves which innervate the visceral and vascular systems. J Physiol. 7:1–80. DOI: 10.1113/jphysiol.1886.sp000207. PMID: 16991419. PMCID: PMC1484971.

29. Iwanaga J, Singh V, Ohtsuka A, Hwang Y, Kim HJ, Moryś J, Ravi KS, Ribatti D, Trainor PA, Sañudo JR, Apaydin N, Şengül G, Albertine KH, Walocha JA, Loukas M, Duparc F, Paulsen F, Del Sol M, Adds P, Hegazy A, Tubbs RS. 2021; Acknowledging the use of human cadaveric tissues in research papers: recommendations from anatomical journal editors. Clin Anat. 34:2–4. DOI: 10.1002/ca.23671. PMID: 32808702.

30. Iwanaga J, Singh V, Takeda S, Ogeng'o J, Kim HJ, Moryś J, Ravi KS, Ribatti D, Trainor PA, Sañudo JR, Apaydin N, Sharma A, Smith HF, Walocha JA, Hegazy AMS, Duparc F, Paulsen F, Del Sol M, Adds P, Louryan S, Fazan VPS, Boddeti RK, Tubbs RS. 2022; Standardized statement for the ethical use of human cadaveric tissues in anatomy research papers: recommendations from Anatomical Journal Editors-in-Chief. Clin Anat. 35:526–8. DOI: 10.1002/ca.23849. PMID: 35218594.
Fig. 1
Schematic drawing of the right side of the neck demonstrating the superior cervical ganglion and its deep branch, the laryngopharyngeal nerve (By David Fisher).
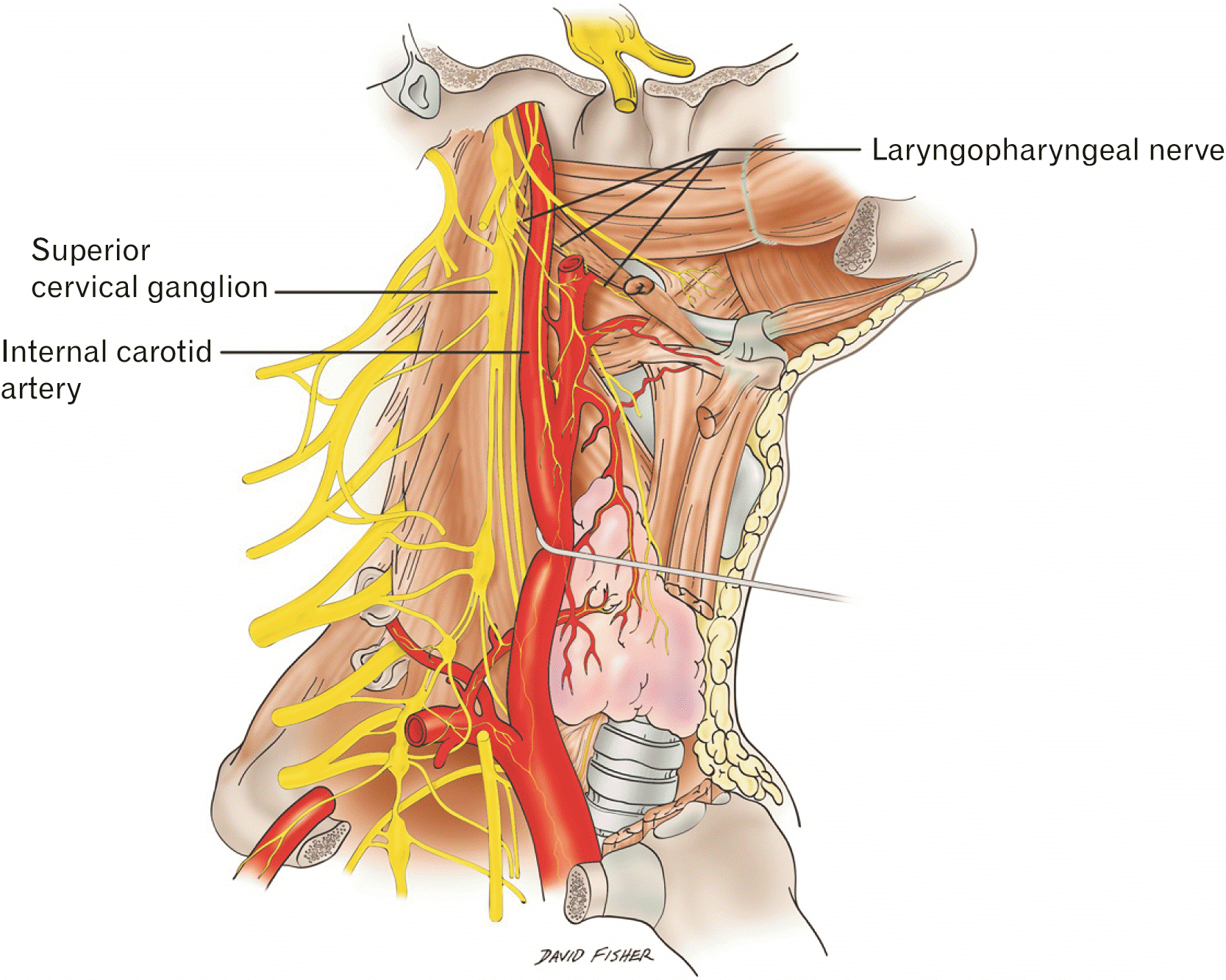
Fig. 2
Schematic drawing of the right side of the neck with structures superficial to the superior cervical ganglion removed for clarity. Note the laryngopharyngeal branches (*) and their ascending (circle) and descending pathways. The ascending branches are thought to terminate primarily in the pharyngeal plexus. A carotid body branch from the laryngopharyngeal nerve is also shown. STA, superficial thyroid artery (By David Fisher).
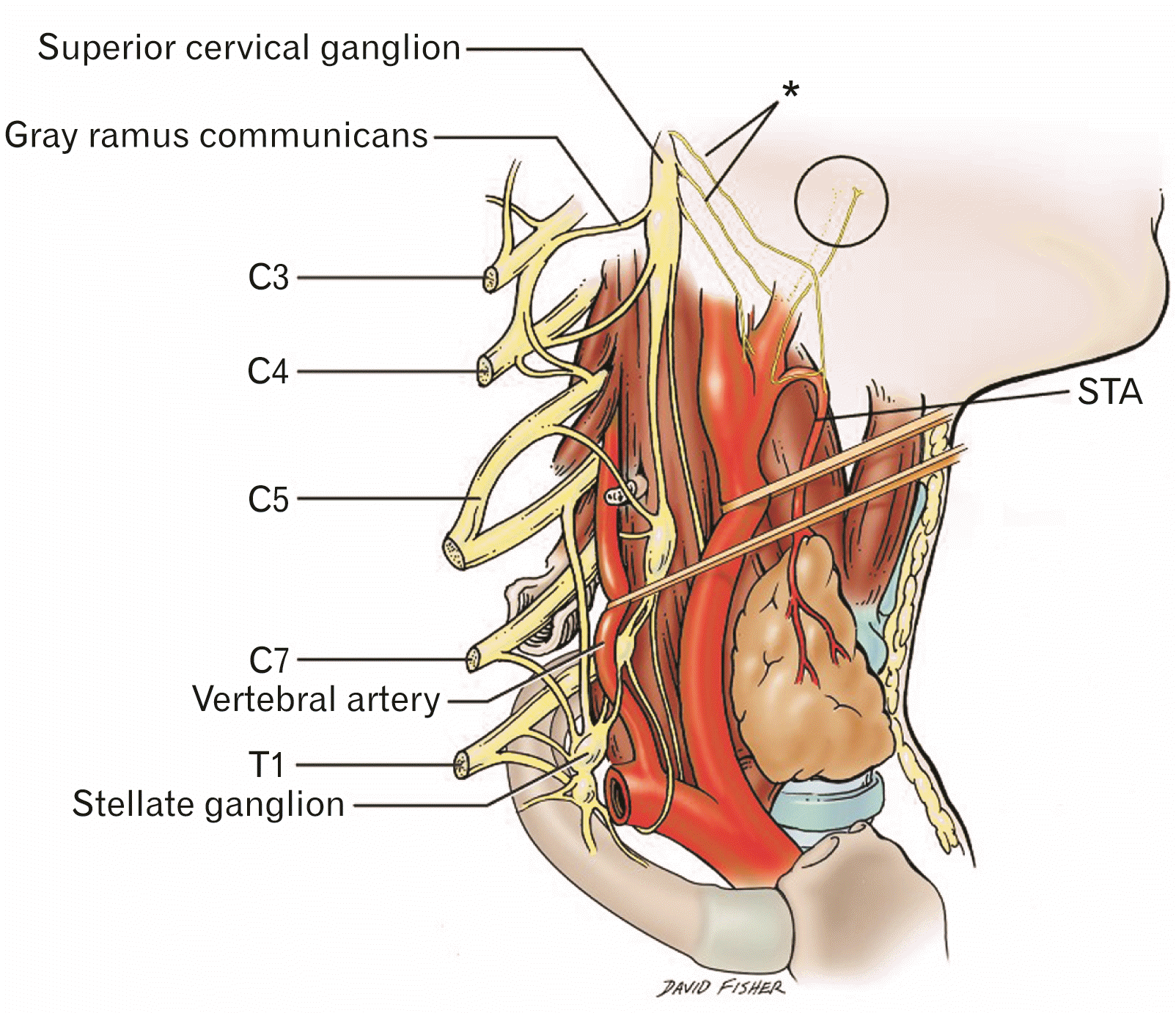
Fig. 3
Cadaveric dissection of the left skull base and opening of the jugular foramen and hypoglossal canal. Note the laryngopharyngeal branch (white arrows) of the superior cervical ganglion (SCG). The IX-XI and XII cranial nerves are seen. Distally, the pharyngeal branch of the IX cranial nerve is shown at the blue arrow and the pharyngeal branch of the X cranial nerve at the yellow arrow. VA, vertebral artery (cadaveric dissection from Tulane University).
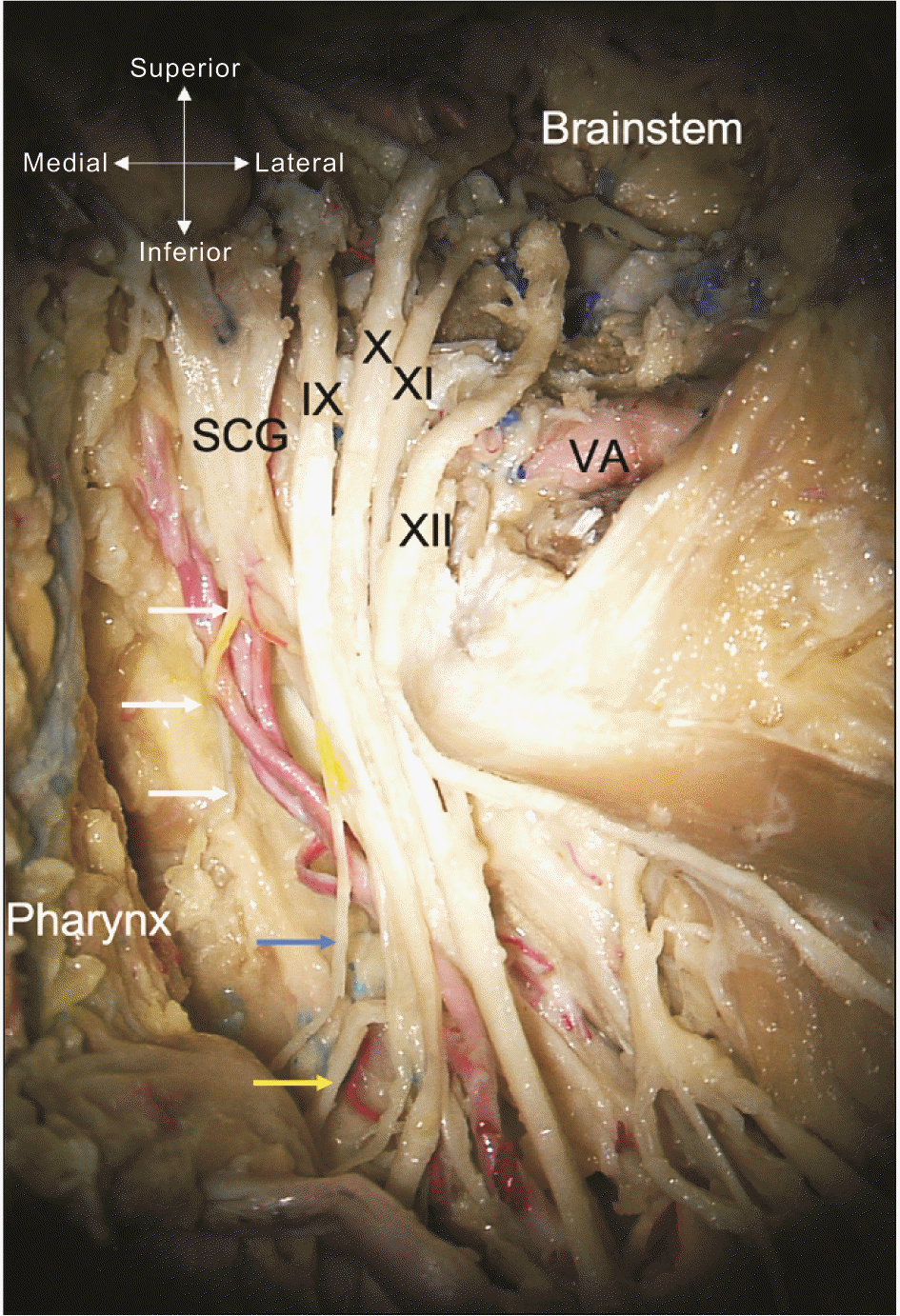
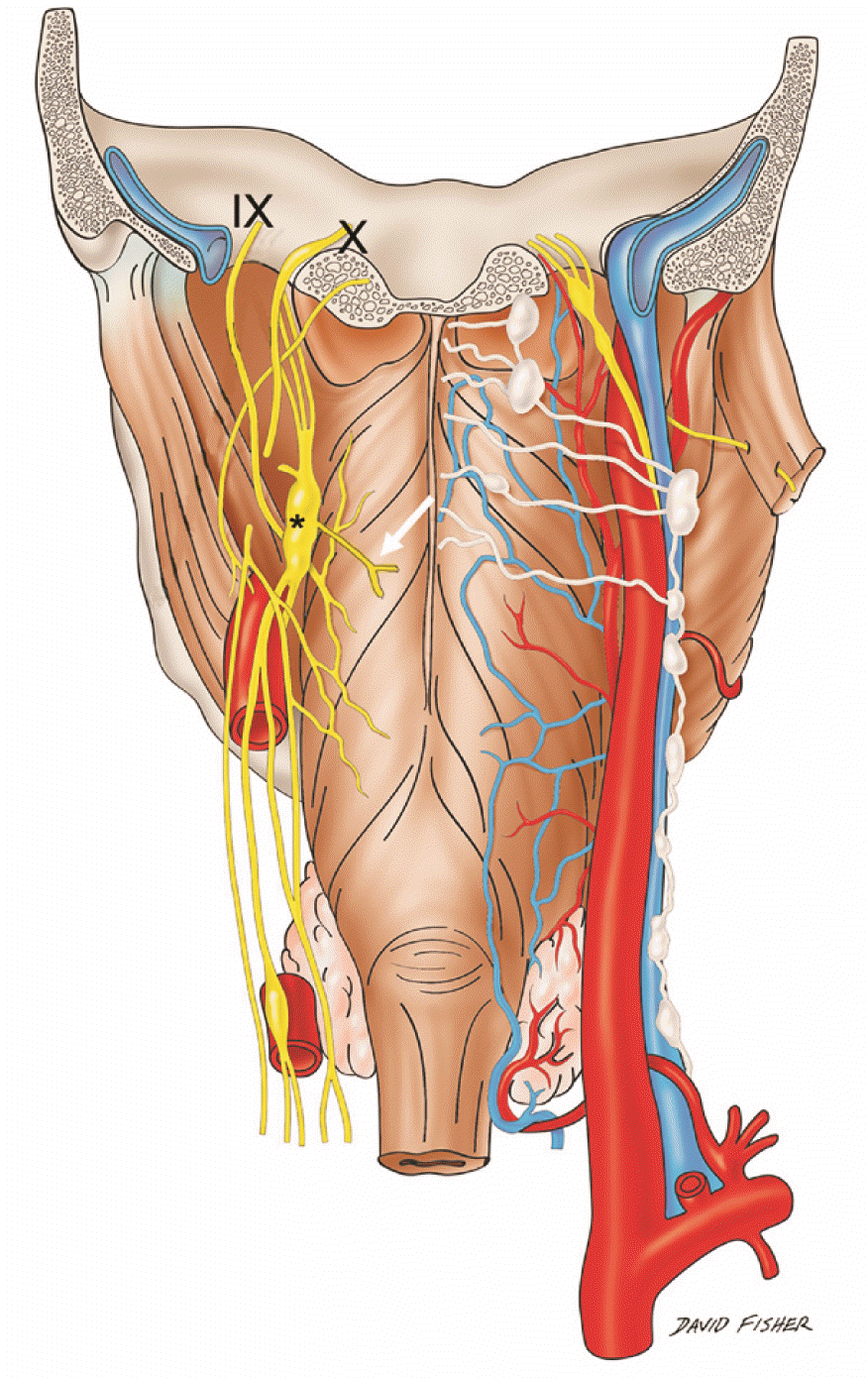
Fig. 4
Schematic drawing of the posterior pharynx and pharyngeal plexus. Note the left laryngopharyngeal branch (white arrow) of the superior cervical ganglion (*). The plexus is seen being contributed to by the pharyngeal branches of the glossopharyngeal (IX) and vagus (X) nerves as well as the laryngopharyngeal nerve (By David Fisher).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download