Abstract
The mentalis muscle is a paired muscle originating from the alveolar bone of the mandible. This muscle is the main target muscle for botulinum neurotoxin (BoNT) injection therapy, which aims to treat cobblestone chin caused by mentalis hyperactivity. However, a lack of knowledge on the anatomy of the mentalis muscle and the properties of BoNT can lead to side effects, such as mouth closure insufficiency and smile asymmetry due to ptosis of the lower lip after BoNT injection procedures. Therefore, we have reviewed the anatomical properties associated with BoNT injection into the mentalis muscle. An up-to-date understanding of the localization of the BoNT injection point according to mandibular anatomy leads to better injection localization into the mentalis muscle. Optimal injection sites have been provided for the mentalis muscle and a proper injection technique has been described. We have suggested optimal injection sites based on the external anatomical landmarks of the mandible. The aim of these guidelines is to maximize the effects of BoNT therapy by minimizing the deleterious effects, which can be very useful in clinical settings.
Botulinum neurotoxin (BoNT), produced by Clostridium botulinum, prevents the release of acetylcholine at the neuromuscular junction and blocks muscular contraction [1, 2] BoNT is frequently used to target the mentalis muscle and diminish the appearance of the “cobblestone” or dimpled chin (Fig. 1A). The cobblestone chin results from hyperactivity of the mentalis muscle as the muscle inserts several fibers in the dermis of the chin.
Given that the mentalis muscle elevates the chin and lower lip, hyperactivity of the mentalis muscle appears in the shortened chin. BoNT injections relax the mentalis muscle, protruding the chin and leading to a more refined appearance of the chin.
The mentalis muscle is also related to the aging process as it produces a mental crease (MC) and an “orange-peel” appearance (when relaxed) on the chin. The MC is the deep groove between the lower lip and the chin prominence, formed by aging and repetitive use of the mentalis muscle. When a cobblestone appearance settles into static wrinkles, the chin can display an orange-peel appearance, with atrophy of the fat tissue and dermal collagen loss. BoNT injections in the mentalis muscle can be used to compensate for and delay this aged appearance.
BoNT injections to treat the cobblestone chin are associated with complications (such as mouth dysfunction with ptosis of the lower lip), often due to a lack of information regarding anatomical factors involved in the procedure [3]. Complications occur when BoNT is injected or diffused into muscles other than the mentalis, particularly the orbicularis oris and depressor labii inferioris. To prevent undesirable results, anatomical-based precise injection in the mentalis muscle and primary treatment with a lower quantity of BoNT are recommended.
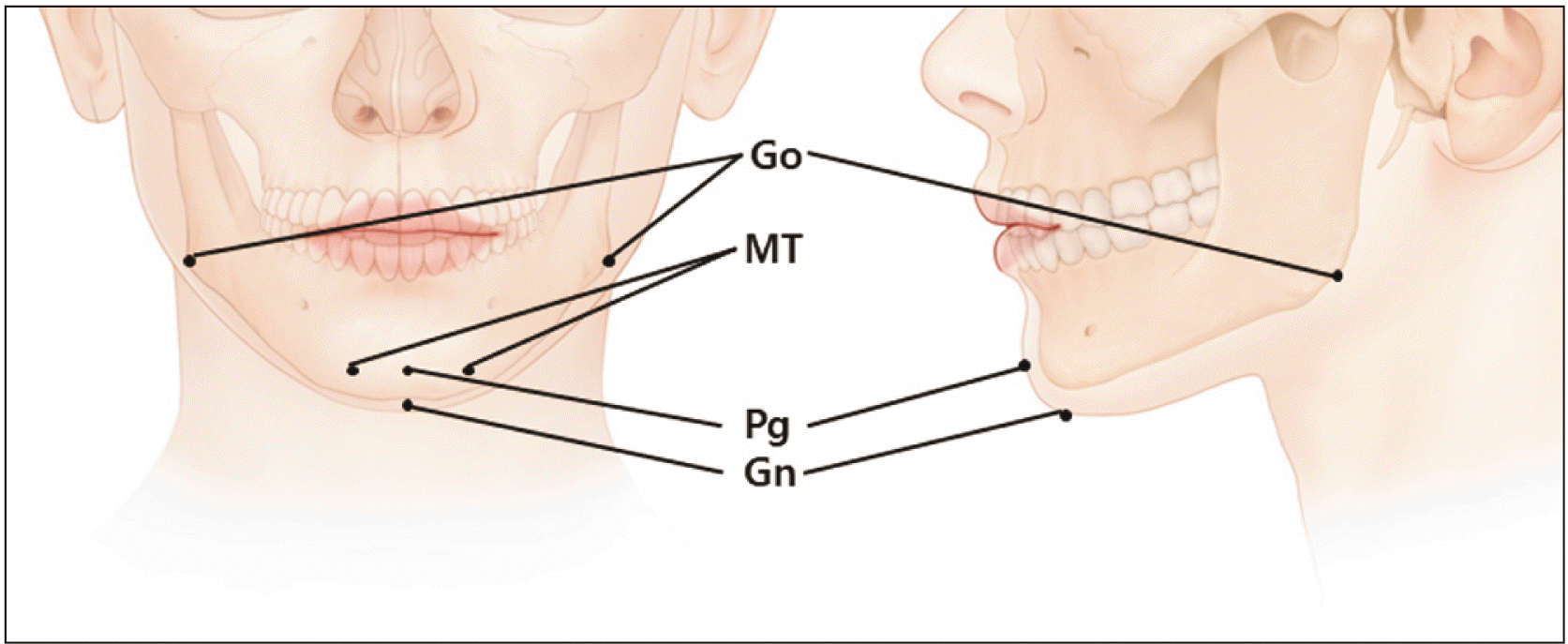
Another factor to consider is the effect of BoNT treatments. Consecutive, short-term injections of BoNT create antibodies that result in poor treatment outcomes [4-7]. Recent anatomical studies have administered BoNT injections in specific locations of particular muscles on the basis of external anatomical landmarks (Fig. 2A) [8-30].
Clinicians treating lower facial rhytides must require a good understanding of the anatomy of the mentalis muscle; however, few anatomical findings regarding BoNT injection in the mentalis muscle have been reviewed with clinical application based on location, injection technique, and dosage.
In this study, we reviewed the anatomical morphology and location of the mentalis muscle to determine the optimal landmarks for guiding BoNT into the mentalis muscle (Fig. 2).
The mentalis muscle fiber originates from the alveolar bone of the anterior mandible (2 cm inferior to the intercheilion line) The anterior mandible is inferior to the lateral incisors and inserts into the dermal layer of the chin. The mentalis originates roughly 0.8 cm lateral to the midline of the chin. The insertion of the mentalis muscle is closer to the midline of the chin than to the origin of the muscle itself. Usually, both sides of the muscle bellies are separated, and fatty tissue lies between their separate origins [31-33].
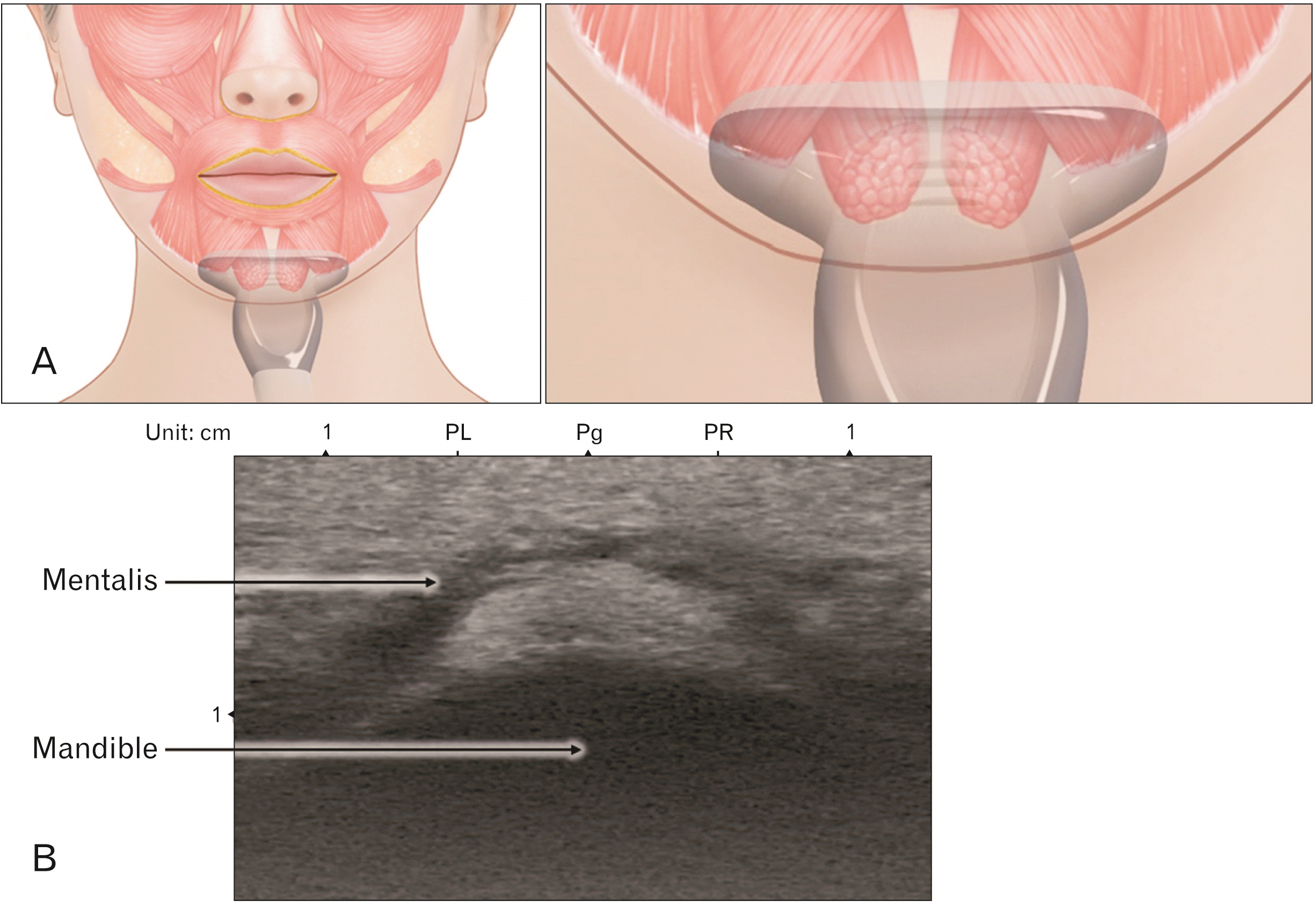
As the muscle fiber is attached to the skin, contraction of the muscle forms MCs at the chin. The mentalis muscle consists of medial, lateral, upper, and lower fibers. The medial fibers, found on both sides of the mentalis muscle, descend anteromedially in a dome-shaped manner (Fig. 1B). The lateral fibers descend obliquely and attach to the skin ipsilaterally. The lateral fibers partially intermingle with the depressor labii inferioris muscles [32]. Lastly, the upper fibers run horizontally and connect to the inferior margin of the orbicularis oris. The upper fibers’ connections act to elevate the lips. Each side of the mentalis muscle belly has an average length and width of 2.0 and 1.2 cm, respectively [32]. Fig. 3 presents an ultrasonographic image of the mentalis muscle observed in the hypoechoic band.
The type of the BoNT used was ona-botulinum toxin-A. The author diluted with 2.5 ml of normal saline to use at a concentration of 4 U/0.1 ml. Large dilution volumes are associated with easier diffusion to the surrounding tissue.
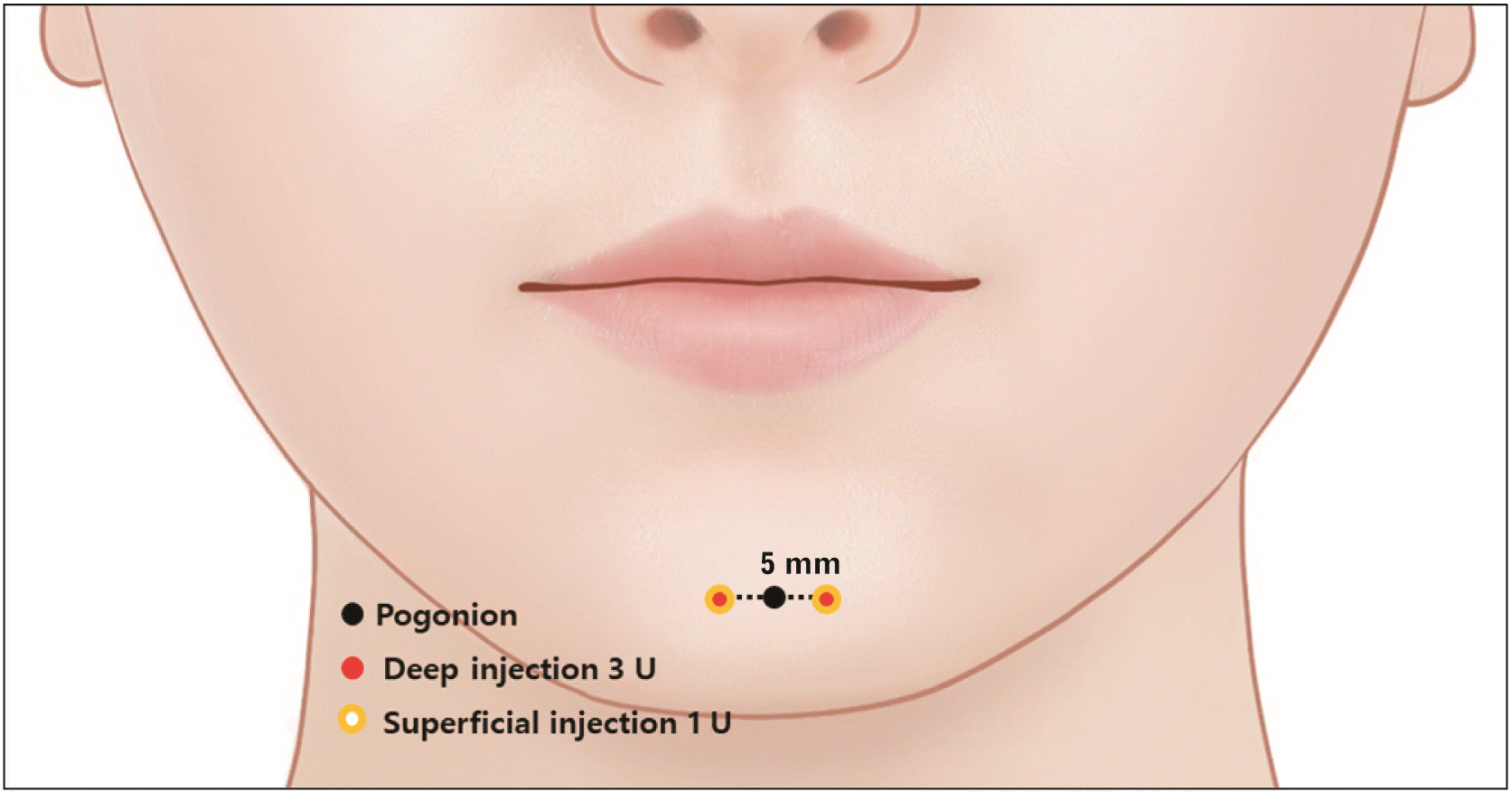
At each point, a deep injection of 3 U per side is injected 0.5 cm lateral to the pogonion (Pg). A superficial injection of 1 U per side is performed at the same injection point (Fig. 4). Injection of BoNT at the upper and lateral sides may affect the orbicularis oris and depressor labii inferioris muscles, leading to asymmetric expression changes of the lower lip or mouth dysfunction with ptosis of the lower lip [32]. Intramuscular and subdermal injections are recommended according to injection depth. The mentalis muscle has a deep muscle belly. The distance between the periosteum and mentalis muscle is 1.1 mm. After touching the mandibular bone, the needle should be slightly retracted, and 3 U of BoNT must then be injected for deep injection. After deep injection and retracting the needle, further subdermal injection of 1 U is recommended. The injection should be performed gently and slowly to prevent the diffusion of BoNTs [34].
Understanding of the three-dimensional morphology of facial expression muscles is necessary because the muscles are densely located in the lower face with layers without fascial structures insertion and origins. Therefore, when BoNT injections target facial muscles, clinicians should consider the delicate anatomical information of the target muscle and the adjacent muscles as well as the positional relationships of these structures.
BoNT is injected into the mentalis muscle to ease or prevent the appearance of a cobblestone chin. However, anatomical considerations for this procedure have not been well described, despite the possibility of side effects resulting from this procedure. Targeting the mentalis muscle with BoNT can cause problems (such as asymmetrical ptosis of the lower lip and dysfunctional mouth opening), which can be critical to patients [35, 36].
In treating the cobblestone appearance of the chin, BoNT diffusion does not often affect nearby muscles such as the orbicularis oris muscle and depressor labii inferioris muscle. Injection of BoNT above and laterally targeting mentalis muscle, however, may affect orbicularis oculi and depressor labii inferioris muscles. Diffusion of BoNT to these muscles may lead to asymmetric expression changes in lower facial expressions [32]. Lower facial muscles intermingle with each other and are duplicated on either side of the face; therefore, BoNT diffusion should be carefully considered during injection procedures.
Yu et al. [37] reported a patient who received BoNT injection in the mentalis muscle, which caused paradoxical bulging. The authors speculate that, when the injection of BoNT fully covers the whole thickness of the muscle, paradoxical bulging may occur. In cases of severe mentalis hyperactivity, an anatomical-based injection is required to prevent imbalanced paralysis and paradoxical chin bulging. Paradoxical bulging occurs when BoNT is unevenly injected [34].
Previous studies have proposed different injection points for the mentalis muscle. Louran [35] suggested superficial injection into the upper part of the muscle. Carruthers and Carruthers suggested the distal-most point from the orbicularis oris muscle to the mentalis muscle as an injection point [38]. Choi et al. [3] found that the mentalis muscle was located 5–10 mm from the midsagittal line and 20–30 mm inferior to the intercheilion line.
The researchers suggested that the area 5 mm lateral to the Pg had the muscle presentation with 4 mm depth of the muscle belly. At this point, the depth of the mentalis muscle to the skin was, on average, 6.7 mm deep. The mean thickness of the muscle was 4 mm, and the length of the muscle to the periosteum was 1.1 mm. The researchers’ results indicate that deep injection is important for targeting the mentalis muscle. However, the cobblestone appearance of the chin is due to the muscle fibers that attach to the skin of the chin; therefore, deep injection alone is insufficient in treating the cobblestone appearance. Superficial BoNT injection should thus be used in conjunction with deep injection to reduce the appearance of the cobblestone chin.
The practice of accurate injection points allows for lower doses of BoNT to be used during injection procedures. To achieve this, broad and thorough anatomical knowledge of the mentalis muscle is crucial. If the preferred clinical result is not achieved, further treatment should be conducted. In summary, deep and superficial injections should be administered at each injection point, lateral to the Pg. In deep injections (3 U of BoNT), after touching the mandibular bone, the needle should be slightly retracted. For superficial injections (1 U of BoNT), after needle retraction from deep injection, a further subdermal injection of 1 U is recommended. With suggested technique, BoNT can affect in resolving MC since the mentalis contraction makes chin moving upward as well as cobblestone appearance for affecting dermal attached muscle fibers.
Acknowledgements
This study was conducted in compliance with the Declaration of Helsinki. Consent was received from the families of the deceased patients before beginning the dissection. The authors sincerely thank those who donated their bodies to science for anatomical research. The results of such research can aid in mankind’s overall knowledge, which can improve patient care. Therefore, these donors and their families deserve the greatest gratitude.
Notes
Author Contributions
Conceptualization: KHY, HJK. Data acquisition: HB. Data analysis or interpretation: HJP, HWH. Drafting of the manuscript: KL. Critical revision of the manuscript: JHL. Approval of the final version of the manuscript: all authors.
Conflicts of Interest
The authors have all considered the conflict of interest statement included in “Author Guidelines”. To the best of our knowledge, no aspect of the authors’ current personal or professional life might significantly affect the views presented on this manuscript. The authors declare no conflicts of interest.
References
1. Dessy LA, Mazzocchi M, Rubino C, Mazzarello V, Spissu N, Scuderi N. 2007; An objective assessment of botulinum toxin A effect on superficial skin texture. Ann Plast Surg. 58:469–73. DOI: 10.1097/01.sap.0000244968.16977.1f. PMID: 17452827.

2. Childers MK. 2004; Targeting the neuromuscular junction in skeletal muscles. Am J Phys Med Rehabil. 83(10 Suppl):S38–44. DOI: 10.1097/01.PHM.0000141129.23219.42. PMID: 15448576.

3. Choi DY, Bae H, Bae JH, Kim HJ, Hu KS. 2021; Effective locations for injecting botulinum toxin into the mentalis muscle; cadaveric and ultrasonographic study. Toxins (Basel). 13:96. DOI: 10.3390/toxins13020096. PMID: 33514053. PMCID: PMC7911364. PMID: 2ffba7fe60e6421aa208765f6742f57e.

4. Hsu TS, Dover JS, Arndt KA. 2004; Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch Dermatol. 140:1351–4. DOI: 10.1001/archderm.140.11.1351. PMID: 15545544.
5. Kinnett D. 2004; Botulinum toxin A injections in children: technique and dosing issues. Am J Phys Med Rehabil. 83(10 Suppl):S59–64. DOI: 10.1097/01.PHM.0000141131.66648.E9. PMID: 15448579.
6. Lepage D, Parratte B, Tatu L, Vuiller F, Monnier G. 2005; Extra- and intramuscular nerve supply of the muscles of the anterior antebrachial compartment: applications for selective neurotomy and for botulinum toxin injection. Surg Radiol Anat. 27:420–30. DOI: 10.1007/s00276-005-0012-9. PMID: 16308665.

7. Pingel J, Nielsen MS, Lauridsen T, Rix K, Bech M, Alkjaer T, Andersen IT, Nielsen JB, Feidenhansl R. 2017; Injection of high dose botulinum-toxin A leads to impaired skeletal muscle function and damage of the fibrilar and non-fibrilar structures. Sci Rep. 7:14746. DOI: 10.1038/s41598-017-14997-3. PMID: 29116170. PMCID: PMC5677119.

8. Yi KH, Choi YJ, Cong L, Lee KL, Hu KS, Kim HJ. 2020; Effective botulinum toxin injection guide for treatment of cervical dystonia. Clin Anat. 33:192–8. DOI: 10.1002/ca.23430. PMID: 31301235.

9. Yi KH, Cong L, Bae JH, Park ES, Rha DW, Kim HJ. 2017; Neuromuscular structure of the tibialis anterior muscle for functional electrical stimulation. Surg Radiol Anat. 39:77–83. DOI: 10.1007/s00276-016-1698-6. PMID: 27206542.

10. Yi KH, Rha DW, Lee SC, Cong L, Lee HJ, Lee YW, Kim HJ, Hu KS. 2016; Intramuscular nerve distribution pattern of ankle invertor muscles in human cadaver using sihler stain. Muscle Nerve. 53:742–7. DOI: 10.1002/mus.24939. PMID: 26467315.

11. Rha DW, Yi KH, Park ES, Park C, Kim HJ. 2016; Intramuscular nerve distribution of the hamstring muscles: application to treating spasticity. Clin Anat. 29:746–51. DOI: 10.1002/ca.22735. PMID: 27213466.

12. Yi KH, Lee HJ, Lee JH, Lee KL, Kim HJ. 2021; Effective botulinum neurotoxin injection in treating iliopsoas spasticity. Clin Anat. 34:431–6. DOI: 10.1002/ca.23670. PMID: 32805076.

13. Yi KH, Lee HJ, Choi YJ, Lee K, Lee JH, Kim HJ. 2021; Anatomical guide for botulinum neurotoxin injection: application to cosmetic shoulder contouring, pain syndromes, and cervical dystonia. Clin Anat. 34:822–8. DOI: 10.1002/ca.23690. PMID: 32996645.

14. Yi KH, Lee KL, Lee JH, Hu HW, Lee K, Seo KK, Kim HJ. 2021; Guidelines for botulinum neurotoxin injections in piriformis syndrome. Clin Anat. 34:1028–34. DOI: 10.1002/ca.23711. PMID: 33347678.

15. Yi KH, Lee HJ, Lee JH, Seo KK, Kim HJ. 2021; Application of botulinum neurotoxin injections in TRAM flap for breast reconstruction: intramuscular neural arborization of the rectus abdominis muscle. Toxins (Basel). 13:269. DOI: 10.3390/toxins13040269. PMID: 33918558. PMCID: PMC8070362. PMID: 15169c322e5c4f158142057c4d95936b.

16. Yi KH, Lee JH, Lee DK, Hu HW, Seo KK, Kim HJ. 2021; Anatomical locations of the motor endplates of sartorius muscle for botulinum toxin injections in treatment of muscle spasticity. Surg Radiol Anat. 43:2025–30. DOI: 10.1007/s00276-021-02813-7. PMID: 34378107. PMCID: PMC8354843.

17. Yi KH, Lee HJ, Seo KK, Kim HJ. 2022; Botulinum neurotoxin injection guidelines regarding flap surgeries in breast reconstruction. J Plast Reconstr Aesthet Surg. 75:503–5. DOI: 10.1016/j.bjps.2021.09.081. PMID: 34776389.

18. Yi KH, Lee JH, Hu HW, Kim HJ. 2022; Novel anatomical guidelines on botulinum neurotoxin injection for wrinkles in the nose region. Toxins (Basel). 14:342. DOI: 10.3390/toxins14050342. PMID: 35622589. PMCID: PMC9144745. PMID: 95ea3c07c66d4527b5265c5ec719f652.

19. Yi KH, Lee JH, Kim HJ. 2022; Intramuscular neural distribution of the serratus anterior muscle: regarding botulinum neurotoxin injection for treating myofascial pain syndrome. Toxins (Basel). 14:271. DOI: 10.3390/toxins14040271. PMID: 35448880. PMCID: PMC9033065. PMID: c4ef8fa6e12442a990c0be9185a526e5.

20. Yi KH, Lee JH, Hu HW, Kim HJ. 2022; Anatomical proposal for botulinum neurotoxin injection for glabellar frown lines. Toxins (Basel). 14:268. DOI: 10.3390/toxins14040268. PMID: 35448877. PMCID: PMC9032255. PMID: dd131953c6e14366befdc986e94b15dd.
21. Yi KH, Lee HJ, Seo KK, Kim HJ. 2022; Intramuscular neural arborization of the latissimus dorsi muscle: application of botulinum neurotoxin injection in flap reconstruction. Toxins (Basel). 14:107. DOI: 10.3390/toxins14020107. PMID: 35202134. PMCID: PMC8878018. PMID: 0c7534ebd76b4902bff96b4bfe81c6b3.

22. Lee HJ, Lee JH, Yi KH, Kim HJ. 2022; Nov. 25. Sonoanatomy and an ultrasound scanning protocol of the intramuscular innervation pattern of the infraspinatus muscle. Reg Anesth Pain Med. [Epub]. https://doi.org/10.1136/rapm-2022-103682. DOI: 10.1136/rapm-2022-103682. PMID: 36427902.

23. Lee HJ, Lee JH, Yi KH, Kim HJ. 2022; Intramuscular innervation of the supraspinatus muscle assessed using Sihler's staining: potential application in myofascial pain syndrome. Toxins (Basel). 14:310. DOI: 10.3390/toxins14050310. PMID: 35622557. PMCID: PMC9143847. PMID: 34b169cd96874bde9b8c4748508d0448.

24. Yi KH, Lee HJ, Choi YJ, Lee JH, Hu KS, Kim HJ. 2020; Intramuscular neural distribution of rhomboid muscles: evaluation for botulinum toxin injection using modified Sihler's method. Toxins (Basel). 12:289. DOI: 10.3390/toxins12050289. PMID: 32375284. PMCID: PMC7291336. PMID: 2a43e00b63b34292bd34bcaa4ce50d09.

25. Yi KH, Lee HJ, Hur HW, Seo KK, Kim HJ. 2022; Guidelines for botulinum neurotoxin injection for facial contouring. Plast Reconstr Surg. 150:562e–71e. DOI: 10.1097/PRS.0000000000009444. PMID: 35759641.

26. Yi KH, Lee JH, Kim GY, Yoon SW, Oh W, Kim HJ. 2022; Novel anatomical proposal for botulinum neurotoxin injection targeting lateral canthal rhytids. Toxins (Basel). 14:462. DOI: 10.3390/toxins14070462. PMID: 35878200. PMCID: PMC9316553. PMID: 46755210c7064a99b625f2d6b6dc6e0a.

27. Yi KH, Lee JH, Kim HM, Kim HJ. 2022; The botulinum neurotoxin for pain control after breast reconstruction: neural distribution of the pectoralis major muscle. Reg Anesth Pain Med. 47:322–6. DOI: 10.1136/rapm-2021-102653. PMID: 35039438.

28. Yi KH, Lee KL, Lee JH, Hu HW, Kim HJ. 2022; Guidance to trigger point injection for treating myofascial pain syndrome: intramuscular neural distribution of the quadratus lumborum. Clin Anat. 35:1100–6. DOI: 10.1002/ca.23918. PMID: 35655442.

29. Yi KH, Lee JH, Lee K, Hu HW, Lee HJ, Kim HJ. 2022; Anatomical proposal for botulinum neurotoxin injection targeting the platysma muscle for treating platysmal band and jawline lifting: a review. Toxins (Basel). 14:868. DOI: 10.3390/toxins14120868. PMID: 36548765. PMCID: PMC9783622. PMID: 1fab78f1f2fb436e8f0adab9755ed1d2.

30. Yi KH, Lee JH, Hur HW, Lee HJ, Choi YJ, Kim HJ. 2023; Jan. 6. Distribution of the intramuscular innervation of the triceps brachii: clinical importance in the treatment of spasticity with botulinum neurotoxin. Clin Anat. [Epub]. https://doi.org/10.1002/ca.24004. DOI: 10.1002/ca.24004. PMID: 36606364.
31. Zide BM, McCarthy J. 1989; The mentalis muscle: an essential component of chin and lower lip position. Plast Reconstr Surg. 83:413–20. DOI: 10.1097/00006534-198903000-00001.

32. Hur MS, Kim HJ, Choi BY, Hu KS, Kim HJ, Lee KS. 2013; Morphology of the mentalis muscle and its relationship with the orbicularis oris and incisivus labii inferioris muscles. J Craniofac Surg. 24:602–4. DOI: 10.1097/SCS.0b013e318267bcc5. PMID: 23524754.

33. Garfein ES, Zide BM. 2008; Chin ptosis: classification, anatomy, and correction. Craniomaxillofac Trauma Reconstr. 1:1–14. DOI: 10.1055/s-0028-1098968. PMID: 22110784. PMCID: PMC3052727.

34. Seo KK. 2017. Botulinum toxin for Asians. Springer;Singapore: DOI: 10.1007/978-981-10-0204-5.
35. Le Louarn C. 2001; Botulinum toxin A and facial lines: the variable concentration. Aesthetic Plast Surg. 25:73–84. DOI: 10.1007/s002660010100. PMID: 11349306.

36. Klein AW. 2004; Contraindications and complications with the use of botulinum toxin. Clin Dermatol. 22:66–75. DOI: 10.1016/j.clindermatol.2003.12.026. PMID: 15158548.

37. Yu N, Liu Y, Chen C, Dong R, Yang E, Wang X. 2020; Paradoxical bulging of mentalis after botulinum toxin type A injection. J Cosmet Dermatol. 19:1290–3. DOI: 10.1111/jocd.13437. PMID: 32346978.

38. Carruthers J, Carruthers A. 2003; Aesthetic botulinum A toxin in the mid and lower face and neck. Dermatol Surg. 29:468–76. DOI: 10.1046/j.1524-4725.2003.29115.x. PMID: 12752513.

Fig. 1
Schematic image of the cobblestone chin. The mental crease also appears marked in the cobblestone chin (A). Schematic image of the mentalis muscle (B). The muscle fiber originates from the alveolar bone of the anterior mandible (2 cm inferior to the intercheilion line), where the structure is inferior to the lateral incisors and inserts to the dermal layer of the chin.
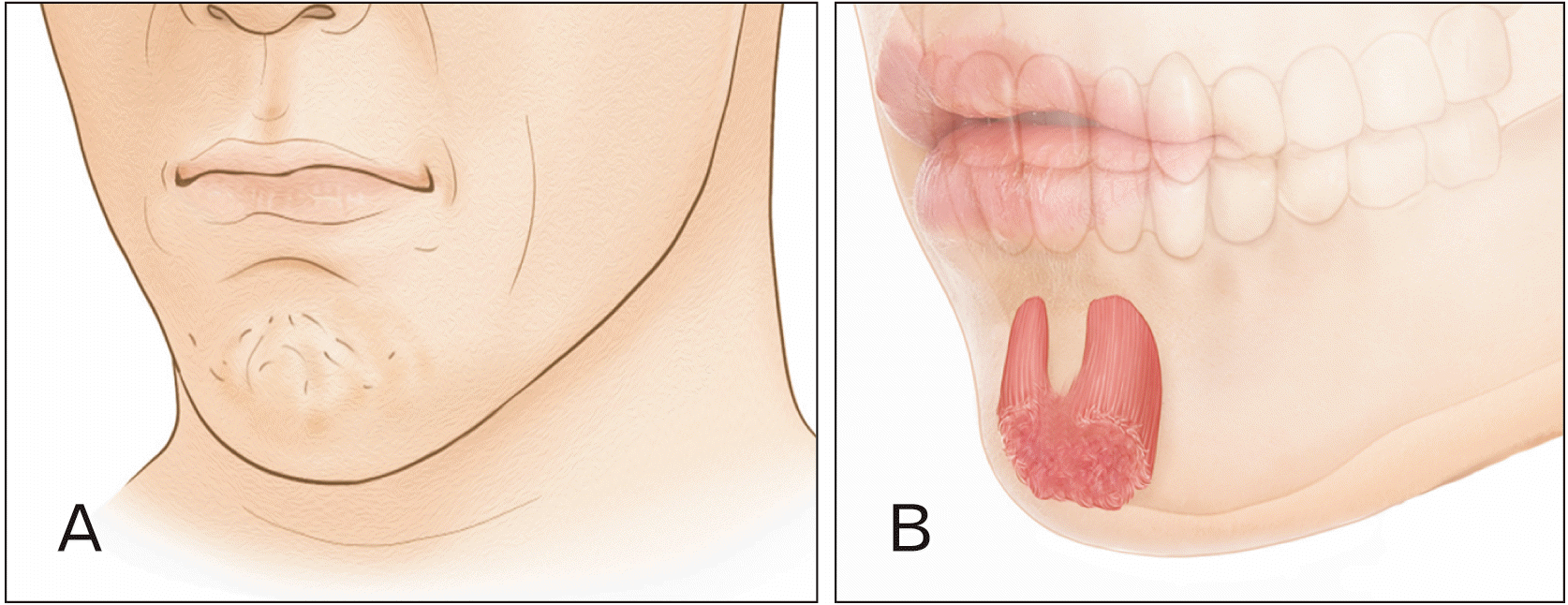
Fig. 2
The external anatomical landmarks of the chin region around depressor anguli oris. Pg, pogonion; Gn, gnathion; MT, mental tubercle; Go, gonion.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download