INTRODUCTION
Children with coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, usually have milder symptoms than adults with this disease [
1-
3]. However, some develop multisystem inflammatory syndrome in children (MIS-C), a serious complication that can occur 2 to 6 weeks after SARS-CoV-2 infection [
2]. MIS-C is characterized by hyperinflammation, multi-organ dysfunction including shock, and infectious trigger (
Table 1) [
1]. With dermatologic involvement, many patients with MIS-C present with ‘Kawasaki disease (KD)-related features’ that constitute the diagnostic criteria for KD [
3]. Accordingly, comparing the clinical and laboratory features of MIS-C and KD has become an important research topic during the COVID-19 pandemic [
3,
4].
KD is a common vasculitis of unknown etiology in children [
5,
6]. In rare cases, KD can present with shock or hypotension (decrease in blood pressure ≥20% from baseline or systolic hypotension for age), which is called KD shock syndrome (KDSS) [
7-
9]. KDSS is more severe than KD without shock in systemic inflammation, coronary artery abnormalities (CAAs), and intravenous immunoglobulin (IVIG) resistance [
10,
11]. Although MIS-C and KDSS share important clinical features such as shock, few studies have compared the two [
8]. Here, we report our experience with MIS-C and KDSS and review the literature to discuss the similarities and differences between the two diseases.
CASE REPORT
Case 1 (MIS-C)
A 9-year-old boy was hospitalized with fever and abdominal pain for 5 days. He had no known medical illness or family history except SARS-CoV-2 infection confirmed by real-time reverse transcription-polymerase chain reaction (RT-PCR) three weeks prior to hospitalization. His height was 135 cm (50-75th percentile) and his weight was 38.3 kg (75-90th percentile). Vital signs were as follows: blood pressure, 74/40 mmHg; respiratory rate, 25 breaths/min; heart rate, 150 beats/min; and body temperature, 37.3°C. Physical examination revealed four KD-related features of maculopapular rash, conjunctival injection, red lips and strawberry tongue, and extremity changes. Important laboratory features included hemoglobin, 10.5 g/dL; white blood cell (WBC) count, 18,060/μL (neutrophil 80%); platelet count, 96,000/μL; erythrocyte sedimentation rate (ESR), 36 mm/h; C-reactive protein (CRP), 82 mg/L (normal <5 mg/L); aspartate transaminase (AST), 51 U/L; alanine transaminase (ALT), 92 U/L; albumin, 2.9 g/dL; international normalized ratio (INR), 1.39 (normal <1.1); fibrinogen, 431 mg/dL; N-terminal prohormone of brain natriuretic peptide (NT-proBNP), 749 pg/mL; and ferritin, 690 ng/mL. Nasopharyngeal swab RT-PCR for SARS-CoV-2 was positive (E/RdRp gene Ct value, 32.09/34.24). Chest X-ray and urinalysis were unremarkable. Echocardiography showed pericardial effusion but no CAAs.
The patient was diagnosed with MIS-C based on multisystem organ involvement (cardiovascular, hematologic, gastrointestinal, and dermatologic), laboratory evidence of inflammation, and evidence of SARS-CoV-2 infection. Fluid and IVIG (2 g/kg/dose) were administered, and his blood pressure (110/70 mmHg) was stable the next day. However, his fever persisted, and he developed oliguria (<0.5 mL/kg/h; blood urea nitrogen [BUN], 25.3 mg/dL; creatinine, 0.6 mg/dL) and generalized edema. Intravenous methylprednisolone (IVMP, 30 mg/kg/day for 5 days), albumin, and diuretics were added to the treatment. His symptoms, including oliguria and edema, improved after 3 days of IVMP treatment, and no subsequent fever was observed. He was discharged on the 9th day of hospitalization. Oral steroids (prednisolone 1 mg/kg) were tapered over 2 weeks and discontinued. During the 6-month follow-up, no further CAAs or recurrence of MIS-C or KD was observed.
Case 2 (KDSS)
A 5-year-old boy was referred to our department due to fever and abdominal pain. The patient had fever and cervical lymphadenopathy for 5 days and was treated with antibiotics for 3 days at the Department of Otorhinolaryngology. However, his symptoms worsened and he developed vomiting, diarrhea, and abdominal pain. He was previously healthy with no specific medical or family history. His height was 118 cm (75-90th percentile) and his weight was 24.7 kg (75-90th percentile). Vital signs were as follows: blood pressure, 90/60 mmHg; respiratory rate, 28 breaths/min; heart rate, 100 beats/min; and body temperature, 38.7°C. Physical examination revealed five KD-related features of urticarial rash, conjunctival injection, red lips and strawberry tongue, cervical lymphadenopathy, and extremity changes. Important laboratory features included hemoglobin, 11.2 g/dL; WBC count, 37,420/μL (neutrophil 93%); platelet count, 103,000/μL; ESR, 59 mm/h; CRP, 205 mg/L; AST, 299 U/L; ALT 181 U/L; albumin, 2.5 g/dL; and INR, 1.51. Chest X-ray and urinalysis were unremarkable. Initial echocardiography showed pericardial effusion and mitral regurgitation without CAAs.
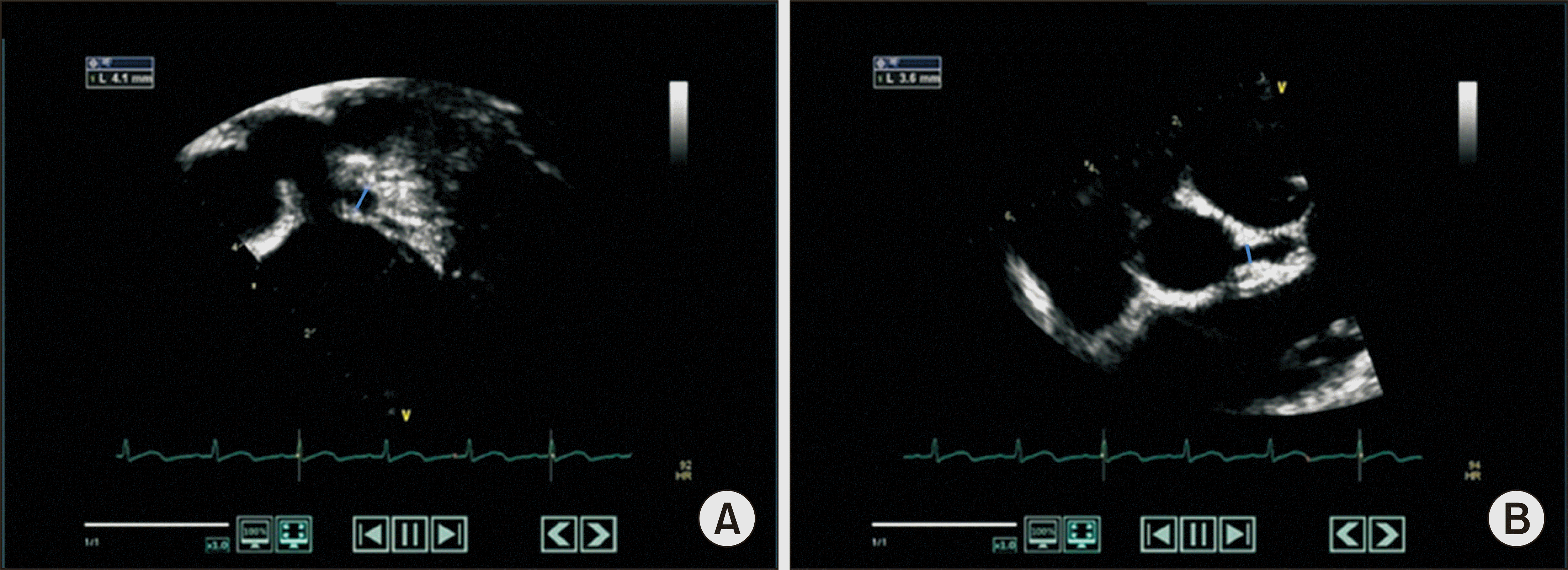
He was diagnosed with typical KD (≥4/5 KD-related features) and administered IVIG (2 g/kg/dose) for treatment. However, fever was uncontrolled with oliguria (<0.5 mL/kg/h) and decreased renal function (BUN, 44.7 mg/dL; creatinine, 1.1 mg/dL). In addition, he developed hypotension (68/30 mmHg) that was unresponsive to fluid therapy. Follow-up echocardiography showed a significant increase in pericardial effusion without CAAs. Considering the possibility of septic shock with acute kidney injury, he was transferred to the intensive care unit (ICU) on the 3rd day of hospitalization. Inotropics (dopamine and norepinephrine), empirical antibiotics (vancomycin and meropenem), IVMP (30 mg/kg/day for 5 days), antithrombin III, albumin, and diuretics were added for treatment. After 6 days of treatment in the ICU, body temperature and blood pressure normalized, and the patient was transferred to the general ward. He was hospitalized for another week to manage scrotal edema and joint pain and was discharged on the 16th day of hospitalization. No pathogens were identified in microbiological studies, including bacterial (blood, urine, and stool cultures) and viral (Epstein-Barr virus [EBV] and multiplex respiratory virus PCR) tests. One week later, outpatient echocardiography revealed a 4.1 mm aneurysm in the left anterior descending (LAD) coronary artery (
Figure 1). Oral steroids (prednisolone 1 mg/kg) were tapered over 3 weeks and discontinued. During the 8-year follow-up, no exacerbation of CAAs and recurrence of KDSS or KD was observed.
DISCUSSION
MIS-C was first reported as hyperinflammatory shock in 8 children in the UK in April 2020 during the COVID-19 pandemic [
12]. Most patients with MIS-C show KD-related features, gastrointestinal problems, and hypotension, as did our two patients in this study. The presence of KD-related features in patients with MIS-C has received clinical attention, but the extent of organ dysfunction in patients with MIS-C is of greater importance as it directly affects the disease course and outcome [
4,
13]. In children and in adults, MIS can occur as a serious post-infectious complication of COVID-19. Chung et al. [
14] have recently reported the first Korean case of MIS in adults (MIS-A). Patients with MIS-C or MIS-A may be misdiagnosed with septic shock due to gastrointestinal infection, as severe abdominal pain and shock are common initial symptoms [
8,
9]. Therefore, MIS-C and MIS-A should be considered as a differential diagnosis in patients with unexplained shock, heart failure, and gastrointestinal symptoms [
14,
15].
Like MIS-C, KDSS has a relatively short history. The term ‘KD shock syndrome’ (KDSS) was first introduced in 2009 to distinguish this severe form of KD from KD without shock [
9]. In actual practice, KDSS shows distinct characteristics in various aspects as KDSS patients have frequent organ dysfunction (gastrointestinal, cardiovascular, hematologic, neurologic, and renal) and more severe systemic inflammation (CRP elevation, hyperferritinemia, anemia, thrombocytopenia, and hypoalbuminemia) [
9-
11]. Epidemiologically, KD and KDSS show different prevalence according to age, region, and race [
6]. Therefore, some experts believe that KDSS should be regarded as a distinct disease entity [
10]. Like MIS-C, KDSS is sometimes confused with septic shock or toxic shock syndrome [
7]. Clinical suspicion of MIS-C or KDSS is a prerequisite for early diagnosis and prompt treatment of the disease [
14,
15].
Here, we describe two cases with KD-related features, systemic inflammation, and organ dysfunction (
Table 1). The overall disease course of these two cases was severe but typical, so the diagnosis itself was not difficult. MIS-C is diagnosed when SARS-CoV-2 is identified in patients with KD-related features and organ dysfunction [
1]. KDSS is diagnosed when shock or hypotension is observed in patients with KD [
9]. The presence or absence of pathogens is not essential for KDSS diagnosis, but various pathogens can be found in KDSS patients (e.g., EBV-confirmed KDSS, mycoplasma-confirmed KDSS, or SARS-CoV-2-confirmed KDSS) [
12,
13]. Case 1 was diagnosed as MIS-C because SARS-CoV-2 was confirmed, and case 2 was diagnosed as KDSS because no pathogen was identified in microbiological studies. MIS-C (case 1) and KDSS (case 2) showed similar clinical manifestations, but the extent of systemic inflammation and organ dysfunction was more severe in KDSS than in MIS-C. Suzuki et al. [
8] reported that MIS-C and KDSS present similar clinical signs and symptoms but differ in severity and are unlikely to be the same disease entity. On the contrary, it has been proposed that MIS-C and KDSS have a common host immune response and are of a single disease spectrum with varying severity [
13]. Regardless of whether MIS-C and KDSS are the same disease entity, at least phenotypically, MIS-C and KDSS share many overlapping features [
3,
4]. We found that the most important factor that differentiates MIS-C from KDSS is whether SARS-CoV-2 has been identified as an infectious trigger. In other words, case 1 (MIS-C) would have been diagnosed as KDSS if there was no evidence of SARS-CoV-2 infection. Similarly, case 2 (KDSS) could have been diagnosed with MIS-C if SARS-CoV-2 was confirmed. The relationship between KDSS and MIS-C is similar to that between pathogen-unidentified septic shock and pneumococcal septic shock (
Figure 2).
Table 2 compares the characteristics of MIS-C cases in the US (n=186), KDSS cases in the literature review (n=103), and KD cases from a nationwide survey in Korea (n=14,916) [
2,
10,
11]. Although KDSS is a subtype of KD, KDSS is closer to MIS-C than to KD in terms of demographics, clinical and laboratory features, organ involvement, and treatment and outcomes. Most patients with KD are younger than 5 years, but two-third of patients with MIS-C and nearly half of patients with KDSS are older than 5 years [
5,
10]. KD is most frequent in East Asia, including Korea, Japan, and Taiwan. However, MIS-C and KDSS are relatively frequent in Europe and North America (i.e., Hispanic and black populations) and rare in East Asia (i.e., Asian populations) [
8,
9]. Systemic inflammation is more severe in MIS-C and KDSS than in KD. Organ involvement, such as gastrointestinal problems or hypotension, rarely occurs in KD but is a principal feature of MIS-C and KDSS. Despite many similarities and the close relationship between MIS-C and KDSS, few studies have addressed the relationship between the two diseases. As this study is a simple case report, large-scale clinical and experimental studies are needed to support our findings. Understanding the similarities and differences between MIS-C and KDSS will be helpful for related research and proper treatment.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download