Abstract
Objective
Bone morphogenetic protein receptor type 2 (BMPR2) has been associated with radiographic changes in ankylosing spondylitis (AS), but further characterization of the cellular signaling pathway in osteoprogenitor (OP) is not clearly understood. The aim of this study was to investigate the expression of BMPR2 and bone morphogenetic protein 2 (BMP2)-mediated responsibility in AS.
Methods
We collected 10 healthy control (HC) and 14 AS-OPs derived from facet joints. Subsequently, we then conducted RNA sequencing with two samples per group and selected BMP-related genes. Facet joint tissues and derived primary OPs were evaluated by validation of selected RNA sequencing data, immunohistochemistry, and comparison of osteogenic differentiation potential.
Results
Based on RNA-sequencing analysis, we found that BMPR2 expression is higher in AS-OPs compared to in HC-OPs. We also validated the increased BMPR2 expression in facet joint tissues with AS and its derived OPs in messenger RNA and protein levels. Additionally, primary AS-OPs showed much greater response to osteogenic differentiation induced by BMP2 and a higher capacity for smad1/5/8-induced RUNX2 expression compared to HCs.
Ankylosing spondylitis (AS) is a common autoinflammatory disease that affects the axial skeleton and is characterized by excessive osteoblast activity and new bone formation at local sites of entheses [1,2]. Clinically, anti-tumor necrosis factor (TNF) and anti-interleukin-17A therapies reduce inflammation in patients with AS, but they do not inhibit radiographic progression [3]. Thus, an understanding of pathological osteoblasts is needed to develop a specific therapeutic target for bony ankylosis in AS patients.
Bone morphogenic proteins (BMPs) are originally identified as growth factor morphogens that are tightly involved in the cascade of skeletal development and bone formation. BMPs can induce diverse signaling pathways in cells. Among these pathways, the BMP-SMAD protein signal is well-known. Upon BMP binding, the serine–threonine kinase activity of type I BMP receptors (BMPRs) fully activates the phosphorylation of smad1/5/8 protein to translocate it to the cell nucleus. Subsequently, the phosphorylated smad1/5/8 protein accumulates to upregulate the transcription level of BMP-response genes. BMPRs forms of type I and 2 type II kinase receptors oligomerize [4]. Many studies suggest that type I BMPRs are a general component of BMP signaling, yet the functional role of type II BMPRs remains largely unknown.
BMP2 is strongly associated with initiation and progression in AS [5-8], and an association between BMP2 variants and susceptibility to AS was reported [9]. Shen et al. put forward the notion that BMP2 has significant effects on the proliferation and differentiation of human mesenchymal stem cells of AS, and upregulated BMPR1A expression triggers fat metaplasia to form new bone in AS [10,11]. Intriguingly, a genetic study revealed that BMPR2 variants are strongly associated with radiographic changes in AS [12]. Additionally, it appears that high expression of BMPR2 in adipocytes has strong osteogenic potency [13]. However, it remains unclear whether extracellular BMP2 agonists discriminate BMPR2 and which smad1/5/8 protein signaling pathways are activated by BMP2 in AS.
This study was carried out in accordance with institutional guidelines and approval from the Ethics Committee of Hanyang University Seoul Hospital with written informed consent provided by participants (IRB 2014-05-002). Facet joints were obtained from 14 patients (all male; mean age, 42.4±8.0 years) diagnosed according to the modified New York criteria [14] and 10 patients (all male; mean age, 56.3±11.2 years) with non-inflammatory spinal diseases as healthy control (HC). The clinical characteristics of human facet joint samples are shown in Table 1.
Facet joints were cut into ≤1 cm bone chips with scissors. The bone chips were washed in serum-free Dulbecco’s modified Eagle medium (DMEM) (SH30243.01; Hyclone Laboratories, Logan, UT, USA) containing 1% penicillin–streptomycin (15140122; Gibco Laboratories, Gaithersburg, MD, USA) antibiotics and incubated in DMEM growth medium containing penicillin–streptomycin and 10% fetal bovine serum (15140122; Gibco Laboratories) at 37°C for 2 weeks to isolate primary OPs. Cell suspensions were filtered through a nylon mesh, washed in serum-free DMEM several times, and seeded for culture. All isolated OPs were assessed with mycoplasma negative using a polymerase chain reaction (PCR)-based method (Takara, 6601).
Two HC-OPs and two AS-OPs were involved in total RNA extraction and RNA sequencing. Data analysis was carried out by EBIOGEN Inc. (Seoul, Korea). Briefly, RNA library construction was performed using the QuantSeq 3’ mRNA-Seq Library Prep Kit (Lexogen, Inc., Greenland, NH, USA) according to the manufacturer's protocol. Analysis of differentially expressed genes andgene ontologieswas carried out using the Microsoft Excel–based Differentially Expressed Gene Analysis (ExDEGA) software package provided by EBIOGEN Inc. Heatmap generation and clustering analysis were performed using R (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria) and MeV (version 4.9.0) for selected genes.
RNA and protein extractions were completed as previously described [15,16]. RNA and proteins were extracted from stimulated cells with Trizol (15596026; Thermo Fisher Scientific, Waltham, MA, USA) and 1× radioimmunoprecipitation assay buffer, respectively. Complementary DNA was generated from 1 μg of total RNA with reverse transcriptase (#EP0442; Thermo Fisher Scientific). The cells were lysed with 1× radioimmunoprecipitation assay buffer containing phosphatase (5870S; Cell Signaling Technology, Danvers, MA, USA) and protease (#535140; Calbiochem, San Diego, CA, USA) inhibitors. Proteins were quantified with a Bradford assay. A total of 30 to 50 μg of protein was subjected to immunoblotting.
We used the following RT-qPCR primers: RUNX2 forward, 5’-TGAGCTGAGAGGACATATGGCC-3’; RUNX2 reverse, 5’-TAGACACCAAACTCCACAGCCC-3’; OCN forward, 5’-ATGAGAGCCCTCACACTCCT-3’; OCN reverse, 5’-CTTGGACACAAAGGCTGCAC-3’; BMPR1A forward, 5’-CAGCATTCGATGGCTGGTTT-3’; BMPR1A reverse, 5’-TCTAAGGACAACAGGCACGC-3’; BMPR1B forward, 5’-GCCCAGTGACCCCTCTTATG -3’; BMPR1B reverse, 5’-GTTTGGGAATGAGGGGCGTA -3’; BMPR2 forward, 5’-CCACTAGAAGGTGGCCGAAC -3’; and BMPR2 reverse, 5’-TCACCTATCTGTATACTGCTGCC -3’; ALK1 forward, 5’-CTCTGCCTACCACCTCCTCT-3’; ALK1 reverse, 5’-CAAGCTGGTGGGCTTGTTTC-3’; ALK2 forward, 5’-AATCCCCGAGACGTGGAGTA-3’; ALK2 reverse, 5’-TTCCCGACACACTCCAACAG-3’.
The antibodies used for immunoblotting and immunofluorescence were as follows: RUNX2 (12556; Cell Signaling Technology), BMPR2 (sc-393304; Santa Cruz Biotechnology, Dallas, TX, USA), ALK1 (AF370; R&D Systems, Minneapolis, MN, USA), phos-smad1/5/8 (sc-12353; Santa Cruz Biotechnology), total-smad1/5/8 (sc-6031-R; Santa Cruz Biotechnology), OPG (sc-390518; Santa Cruz Biotechnology), phos-ERK (5683; Cell Signaling Technology), phos-p38 (9215; Cell Signaling Technology), and GAPDH (2118; Cell Signaling Technology).
Immunohistochemistry was performed as previously described [15,16]. The facet joint samples were fixed in 10% formalin for 1 week, dehydrated, and embedded in paraffin. Tissues were sectioned into 5-μm-thick slices and used for immunohistochemistry for BMPR2. The images were obtained using an Eclipse microscope (Nikon, Tokyo, Japan) with transmitted light at 4× and 20× magnifications.
Osteogenic differentiation and its quantitative methods were previously reported [16-20]. Briefly, primary OPs from the facet joints were seeded in DMEM growth medium (SH30243.01; Hyclone Laboratories) and then differentiated with osteogenic medium (ascorbic acid, β-glycerophosphate, and dexamethasone) into mature osteoblasts for indicated days. Matrix maturation of differentiation was assessed by alkaline phosphatase (ALP) staining and activity. Matrix mineralization were assessed by alizarin red (ARS), von Kossa (VON), and hydroxyapatite (HA) staining. Differentiation medium was changed every 3 days.
Data were generated and analyzed by GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA). Statistical analysis was performed using the Mann–Whitney U test with unpaired tests. Values are shown as mean±standard error of the mean from ≥3 independent experiments. The asterisks represent the level of statistical significance (p<0.05, p<0.01, p<0.001).
To distinguish the basal level of BMP-related genes, we collected 2 HC-OPs and 2 AS-OPs and conducted RNA sequencing. As shown in Figure 1A, the messenger RNA (mRNA) expression of BMP3 and BMP6 was upregulated and downregulated in AS-OPs compared to HC-OPs, respectively. BMPR1A and BMPR1B expression was not different between the OP groups, whereas BMPR2 and ALK1 expressions were obviously elevated in AS-OPs (Figure 1A). We then validated BMPRs using RT-qPCR and revealed that BMPR2 expression, but not BMPR1A, BMPR1B, ALK1, or ALK2 expression, was significantly increased in AS-OPs (Figure 1B). ALK1 protein expression is statically different between HC-OPs and AS-OPs, but an increase in BMPR2 protein expression in AS-OPs was confirmed (Figure 1C). Moreover, BMPR2-expressing bone-lining cells were more abundant and statistically elevated in facet joints with AS (Figure 1D). Collectively, we found that BMPR2 expression is significantly higher in patients with AS compared to HCs.
Since BMP2 is well-known as a trigger for excessive osteoblastic activity in AS, we treated AS-OPs with various BMP2 doses. BMP2 treatment dramatically upregulated the mRNA and protein expression of RUNX2 in AS-OPs (Figure 2A). There were no substantial changes in OPG, phos-ERK, and phos-p38 protein levels, but BMP2 treatment led to an increase in the phos-smad1/5/8 and RUNX2 protein concentrations of AS-OPs in a time-dependent manner as well as a decrease in total-smad1/5/8 and BMPR2 protein concentrations (Figure 2B). Consistent with a previous report [10,21], AS-OPs exhibited greater phosphorylation of smad1/5/8 and sustained BMPR2 and induction of RUNX2 protein in BMP2 treatment compared to HC-OPs (Figure 2C). These results indicate that AS-OPs have a greater capacity for induction of RUNX2 by BMP2 than do HC-OPs.
Finally, we treated both HC-OPs and AS-OPs with BMP2 (50 ng/mL) during osteogenic differentiation and observed bone matrix maturation and matrix mineralization of osteoblasts on indicated days. For matrix maturation, ALP staining and activity was markedly pronounced in AS-OPs compared to control-OPs (Figure 3A and B). For matrix mineralization, ARS, VON, and HA staining and quantification data revealed that BMP2 treatment accelerated matrix mineralization in AS-OPs compared to HC-OPs (Figure 3C and D). As expected, expression of RUNX2 and OCN mRNA was increased to a greater degree in AS-OPs (Figure 3E). Therefore, BMP2 treatment had an additive effect on both matrix maturation and matrix mineralization of AS-OPs compared to HC-OPs.
Long-term follow-up studies covering anti-TNF use have commonly reported reductions in inflammation and alleviation of ankylosis progression [22]. However, this effect cannot fully explain how chronic TNF exposure is related to new bone formation in AS. Thus, uncertainties exist regarding the precise pathogenesis of AS. Surprisingly, Lories et al. [6] highlighted BMP to play a critical role in onset and progression of spinal ankylosis, implicating that clinical application of the BMP axis for new bone formation. In line with the above, our results support the notions that BMPR2 is increased in AS-OPs and is much more sensitive to mediating bone formation activity in AS patients than HCs.
RUNX2 is a master regulator of bone formation and development [23,24]. RUNX2 is known to positively regulate ALP and OCN expression levels [25]. ALP is an early marker and a sign of osteogenesis; OCN is a late osteogenic marker that forms calcified nodule complexes. We previously reported that the ALP basal level is high in AS patients compared to RA and helps promote bone-forming activity [15,16,18,19]. In line with other previous reports, we confirmed that BMP2 is a trigger for RUNX2 expression [25]. BMP2 showed a potent function in accelerating osteoblast differentiation of mesenchymal stem cells derived from AS [10,21]. Our data highlighted that BMPs show substantially low expression in peripheral blood mononuclear cells but relatively high expression in OPs (Supplementary Figure 1).
BMP2 serum level is positively associated with proinflammatory cytokines, high bone-forming activity, and disease progression in AS patients [6,26-29]. Thus, we treated OPs with diverse proinflammatory cytokines such as TNF, interleukin-17A, and interleukin-23 but BMP2 expression in OPs was not altered following these treatments. Additionally, Ding et al. [7] reported that overexpression of BMP2 significantly induced BMPR2 expression in fibroblasts derived from AS, while we revealed that changes in BMPR1A, BMPR1B, and BMP2 mRNA expression did not differ between vehicle and BMP2 treatment groups. As shown in Figure 2, BMP2 treatment reduced BMPR2 protein expression in AS-OPs to induce upregulation of RUNX2 transcript by smad1/5/8 pathway activation. Collectively, AS-OPs showed much higher sensitivity to BMP2 for an increase in RUNX2 by smad1/5/8 signaling, reflecting high bone-forming activity in AS.
It is clear that BMPs and their receptor (ALK2; activin type I receptor) have a distinct role in promoting bone formation in AS [5]. Intriguingly, Breban et al. suggested that genetic HLA-B27 specifically interacts with BMPRs and influences the phosphorylation of SMAD proteins, which leads inflammation and ossification in AS development [30,31]. In addition, the antagonist of BMPRs exerts inhibitory functions on pathological bone features in mouse and Drosophila models of AS [30,32,33]. Less is known about the functional role of BMPR2 in AS. However, our results provide additional insight into BMP-mediated pathological bone features such as new bone formation, promoting ossification, and accelerated bone-forming activity.
This study has several limitations. First, the exact mechanisms behind the induction of the smad1/5/8-RUNX2 pathway are yet to be determined. Second, BMPR is a commonly formed heterodimer, and further molecular characterization and structure complexes of BMPR1A or BMPR1B based on BMPR2 should be determined. Third, it is unclear which stimulant can induce BMP2 expression and BMPR function. Fourth, BMP2K expression was increased in AS-OPs, but functional study remains necessary. Last, the effect of BMPR inhibition on AS in in vitro and in vivo models needs further study.
The most important finding of the current study is that BMPR2 is strongly expressed in AS-OPs, albeit with a stronger tendency towards osteoblast differentiation and bone-forming activity through smad1/5/8-RUNX2 regulation in response to BMP2 stimulation. Additionally, cytological observation revealed that BMPR2 is predominantly expressed in bone-lining cells of AS patients compared to HCs.
Supplementary data can be found with this article online at https://doi.org/10.4078/jrd.2023.0024.
Notes
REFERENCES
1. Mauro D, Thomas R, Guggino G, Lories R, Brown MA, Ciccia F. 2021; Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat Rev Rheumatol. 17:387–404. DOI: 10.1038/s41584-021-00625-y. PMID: 34113018.

2. Inman RD. 2021; Axial spondyloarthritis: current advances, future challenges. J Rheum Dis. 28:55–9. DOI: 10.4078/jrd.2021.28.2.55. PMID: 37476012. PMCID: PMC10324891.

3. Deodhar A. 2018; Spondyloarthropathies: TNF inhibitors and structural damage in ankylosing spondylitis. Nat Rev Rheumatol. 14:5–6. DOI: 10.1038/nrrheum.2017.197. PMID: 29213122.

4. Karim MS, Madamanchi A, Dutko JA, Mullins MC, Umulis DM. 2021; Heterodimer-heterotetramer formation mediates enhanced sensor activity in a biophysical model for BMP signaling. PLoS Comput Biol. 17:e1009422. DOI: 10.1371/journal.pcbi.1009422. PMID: 34591841. PMCID: PMC8509922.

5. Carter S, Braem K, Lories RJ. 2012; The role of bone morphogenetic proteins in ankylosing spondylitis. Ther Adv Musculoskelet Dis. 4:293–9. DOI: 10.1177/1759720X12444175. PMID: 22859928. PMCID: PMC3403253.

6. Lories RJ, Derese I, Luyten FP. 2005; Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 115:1571–9. DOI: 10.1172/JCI23738. PMID: 15902307. PMCID: PMC1090472.

7. Ding L, Yin Y, Hou Y, Jiang H, Zhang J, Dai Z, et al. 2021; microRNA-214-3p suppresses ankylosing spondylitis fibroblast osteogenesis via BMP-TGFβ axis and BMP2. Front Endocrinol (Lausanne). 11:609753. DOI: 10.3389/fendo.2020.609753. PMID: 33935961. PMCID: PMC8082363.

8. Chen HA, Chen CH, Lin YJ, Chen PC, Chen WS, Lu CL, et al. 2010; Association of bone morphogenetic proteins with spinal fusion in ankylosing spondylitis. J Rheumatol. 37:2126–32. DOI: 10.3899/jrheum.100200. PMID: 20682677.

9. Qi D, Tian X, Wang Y, Zheng G, Zhang X. 2020; BMP2 variants in the risk of ankylosing spondylitis. J Cell Biochem. 121:3935–40. DOI: 10.1002/jcb.29563. PMID: 31713925.

10. Xie Z, Wang P, Li Y, Deng W, Zhang X, Su H, et al. 2016; Imbalance between bone morphogenetic protein 2 and Noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol. 68:430–40. DOI: 10.1002/art.39433. PMID: 26413886.

11. Liu Z, Wang P, Cen S, Gao L, Xie Z, Wu X, et al. 2019; Increased BMPR1A expression enhances the adipogenic differentiation of mesenchymal stem cells in patients with ankylosing spondylitis. Stem Cells Int. 2019:4143167. DOI: 10.1155/2019/4143167. PMID: 31827527. PMCID: PMC6885782.

12. Cortes A, Maksymowych WP, Wordsworth BP, Inman RD, Danoy P, Rahman P, et al. 2015; Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Ann Rheum Dis. 74:1387–93. DOI: 10.1136/annrheumdis-2013-204835. PMID: 24651623. PMCID: PMC4470170.

13. Skillington J, Choy L, Derynck R. 2002; Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol. 159:135–46. DOI: 10.1083/jcb.200204060. PMID: 12379805. PMCID: PMC2173483.

14. van der Linden S, Valkenburg HA, Cats A. 1984; Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27:361–8. DOI: 10.1002/art.1780270401. PMID: 6231933.
15. Jo S, Lee JS, Nam B, Lee YL, Kim H, Lee EY, et al. 2022; SOX9+ enthesis cells are associated with spinal ankylosis in ankylosing spondylitis. Osteoarthritis Cartilage. 30:280–90. DOI: 10.1016/j.joca.2021.11.013. PMID: 34826571.

16. Jo S, Lee SH, Park J, Nam B, Kim H, Youn J, et al. 2023; Platelet-derived growth factor B is a key element in the pathological bone formation of ankylosing spondylitis. J Bone Miner Res. 38:300–12. DOI: 10.1002/jbmr.4751. PMID: 36422470.

17. Park PR, Jo S, Jin SH, Kim TJ. 2020; MicroRNA-10b plays a role in bone formation by suppressing interleukin-22 in ankylosing spondylitis. J Rheum Dis. 27:61–7. DOI: 10.4078/jrd.2020.27.1.61.

18. Jo S, Han J, Lee YL, Yoon S, Lee J, Wang SE, et al. 2019; Regulation of osteoblasts by alkaline phosphatase in ankylosing spondylitis. Int J Rheum Dis. 22:252–61. DOI: 10.1111/1756-185X.13419. PMID: 30415492.

19. Jo S, Kang S, Han J, Choi SH, Park YS, Sung IH, et al. 2018; Accelerated osteogenic differentiation of human bone-derived cells in ankylosing spondylitis. J Bone Miner Metab. 36:307–13. DOI: 10.1007/s00774-017-0846-3. PMID: 28589411.

20. Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, et al. 2018; IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther. 20:115. DOI: 10.1186/s13075-018-1582-3. PMID: 29880011. PMCID: PMC5992730.

21. Zheng G, Xie Z, Wang P, Li J, Li M, Cen S, et al. 2019; Enhanced osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis: a study based on a three-dimensional biomimetic environment. Cell Death Dis. 10:350. DOI: 10.1038/s41419-019-1586-1. PMID: 31024000. PMCID: PMC6484086.

22. Koo BS, Oh JS, Park SY, Shin JH, Ahn GY, Lee S, et al. 2020; Tumour necrosis factor inhibitors slow radiographic progression in patients with ankylosing spondylitis: 18-year real-world evidence. Ann Rheum Dis. 79:1327–32. DOI: 10.1136/annrheumdis-2019-216741. PMID: 32660979.

23. Lim KE, Park NR, Che X, Han MS, Jeong JH, Kim SY, et al. 2015; Core binding factor β of osteoblasts maintains cortical bone mass via stabilization of Runx2 in mice. J Bone Miner Res. 30:715–22. DOI: 10.1002/jbmr.2397. PMID: 25358268. PMCID: PMC7363154.

24. Gomez-Puerto MC, Iyengar PV, García de Vinuesa A, Ten Dijke P, Sanchez-Duffhues G. 2019; Bone morphogenetic protein receptor signal transduction in human disease. J Pathol. 247:9–20. DOI: 10.1002/path.5170. PMID: 30246251. PMCID: PMC6587955.
25. Kim WJ, Shin HL, Kim BS, Kim HJ, Ryoo HM. 2020; RUNX2-modifying enzymes: therapeutic targets for bone diseases. Exp Mol Med. 52:1178–84. DOI: 10.1038/s12276-020-0471-4. PMID: 32788656. PMCID: PMC8080656.

26. Croes M, Kruyt MC, Groen WM, van Dorenmalen KMA, Dhert WJA, Öner FC, et al. 2018; Interleukin 17 enhances bone morphogenetic protein-2-induced ectopic bone formation. Sci Rep. 8:7269. DOI: 10.1038/s41598-018-25564-9. PMID: 29740080. PMCID: PMC5940874.

27. Park MC, Park YB, Lee SK. 2008; Relationship of bone morphogenetic proteins to disease activity and radiographic damage in patients with ankylosing spondylitis. Scand J Rheumatol. 37:200–4. DOI: 10.1080/03009740701774941. PMID: 18465455.

28. Lories RJ, Derese I, Ceuppens JL, Luyten FP. 2003; Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 48:2807–18. DOI: 10.1002/art.11389. PMID: 14558086.

29. Briolay A, El Jamal A, Arnolfo P, Le Goff B, Blanchard F, Magne D, et al. 2020; Enhanced BMP-2/BMP-4 ratio in patients with peripheral spondyloarthritis and in cytokine- and stretch-stimulated mouse chondrocytes. Arthritis Res Ther. 22:234. DOI: 10.1186/s13075-020-02330-9. PMID: 33046134. PMCID: PMC7552569.

30. Grandon B, Rincheval-Arnold A, Jah N, Corsi JM, Araujo LM, Glatigny S, et al. 2019; HLA-B27 alters BMP/TGFβ signalling in Drosophila, revealing putative pathogenic mechanism for spondyloarthritis. Ann Rheum Dis. 78:1653–62. DOI: 10.1136/annrheumdis-2019-215832. PMID: 31563893.

31. Jah N, Jobart-Malfait A, Ermoza K, Noteuil A, Chiocchia G, Breban M, et al. 2020; HLA-B27 subtypes predisposing to ankylosing spondylitis accumulate in an endoplasmic reticulum-derived compartment apart from the peptide-loading complex. Arthritis Rheumatol. 72:1534–46. DOI: 10.1002/art.41281. PMID: 32270915.
32. Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, et al. 2008; BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 14:1363–9. Erratum in: Nat Med 2009;15:117. DOI: 10.1038/nm.1888. PMID: 19029982. PMCID: PMC2846458.
33. Breban M, Glatigny S, Cherqaoui B, Beaufrère M, Lauraine M, Rincheval-Arnold A, et al. 2021; Lessons on SpA pathogenesis from animal models. Semin Immunopathol. 43:207–19. DOI: 10.1007/s00281-020-00832-x. PMID: 33449154.

Fig. 1
BMPR2 is highly expressed in patients with AS. (A) Two healthy control (HC)-OPs and two AS-OPs conducted RNA sequencing. Heatmap with the BMP-related genes were selected and shown. (B) BMPR1A, BMPR1B, BMPR2, ALK1, and ALK2 expressions were validated by RT-qPCR (HC-OPs: n=8; AS-OPs: n=8). BMPR2 and ALK1 expressions were validated by (C) immunoblotting (HC-OPs: n=2, AS-OPs: n=6) and (D) immunohistochemistry (HC: n=6; AS: n=6). BMPR2-positive cells in bone-lining cells were counted. Representative images are shown from two individual samples per group. (D) The scale bar showed is 200 μm. AS: ankylosing spondylitis, OP: osteoprogenitor. Values are the mean±standard error of the mean. Statistical significance was determined with *p<0.05, **p<0.01, by Mann–Whitney U test.
Fig. 2
AS-OPs had a greater capacity for induction of RUNX2 by BMP2. (A) AS-OPs were treated with exogenous BMP2 as indicated dose for a day and subjected to immunoblotting (upper) and RT-qPCR (lower). (B) AS-OPs were treated with 50 ng/mL BMP2 as indicated time and subjected to immunoblotting. (C) Both HC-OPs and AS-OPs were stimulated with 50 ng/mL BMP2 as indicated time and subjected to immunoblotting. HC: healthy control, AS: ankylosing spondylitis, OP: osteoprogenitor. Values are the mean±standard deviation. Statistical significance was determined with ***p<0.01, by Mann–Whitney U test.
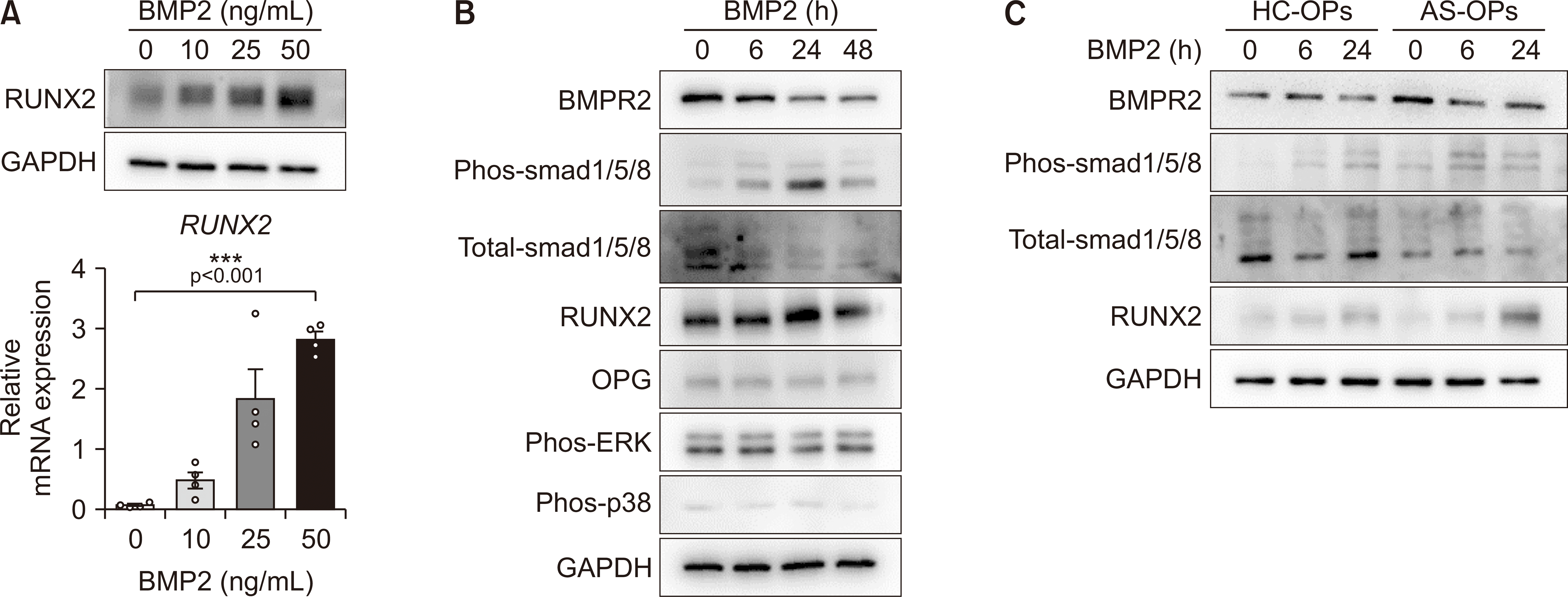
Fig. 3
AS-OPs revealed a greater capacity for RUNX2 induction by BMP2. Both HC-OPs and AS-OPs were stimulated with 50 ng/mL BMP2 as indicated days and subjected to (A) ALP staining at 3 and 7 days, (B) ALP activity, (C) ARS, VON, and HA staining at 21 days, (D) quantification data of ARS, VON, and HA staining, (E) RT-qPCR for RUNX2 and OCN expressions. AS: ankylosing spondylitis, OP: osteoprogenitor, HC: healthy control, ALP: alkaline phosphatase, OM: osteogenic medium. Values are the mean±standard error of the mean. Statistical significance was determined with *p<0.05, **p<0.01, ***p<0.001, by Mann–Whitney U test.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download