Dear Editor,
In the evolving landscape of the medical field, there is a growing emphasis on the significance of standardized records for integrating, managing, and sharing data across multiple institutions [1]. This emphasis is clearly demonstrated through initiatives such as the Health Information Exchange Service promoted by the Korea Health Information Service as well as the growing interest in big data research. Most laboratory test results are uniformly reported through laboratory information systems (LISs), which leads to standardized data [2]. However, data of point-of-care (POC) tests, like point-of-care glucose meter tests (PGMTs), are not standardized [3]. Although many Korean and global medical institutions have adopted computerized reporting to ensure the reliability of PGMT results [4], the extent and scope of this implementation differ substantially depending on the acceptance of data integration and standardization among medical staff. This discrepancy often results in fragmented data that are neither easily accessible nor shareable across the hospital, leading to incomplete or inaccurate patient records [1, 5]. To address these issues and promote more efficient hospital operations and patient care, we share our experience of implementing unified computerized PGMT reporting.
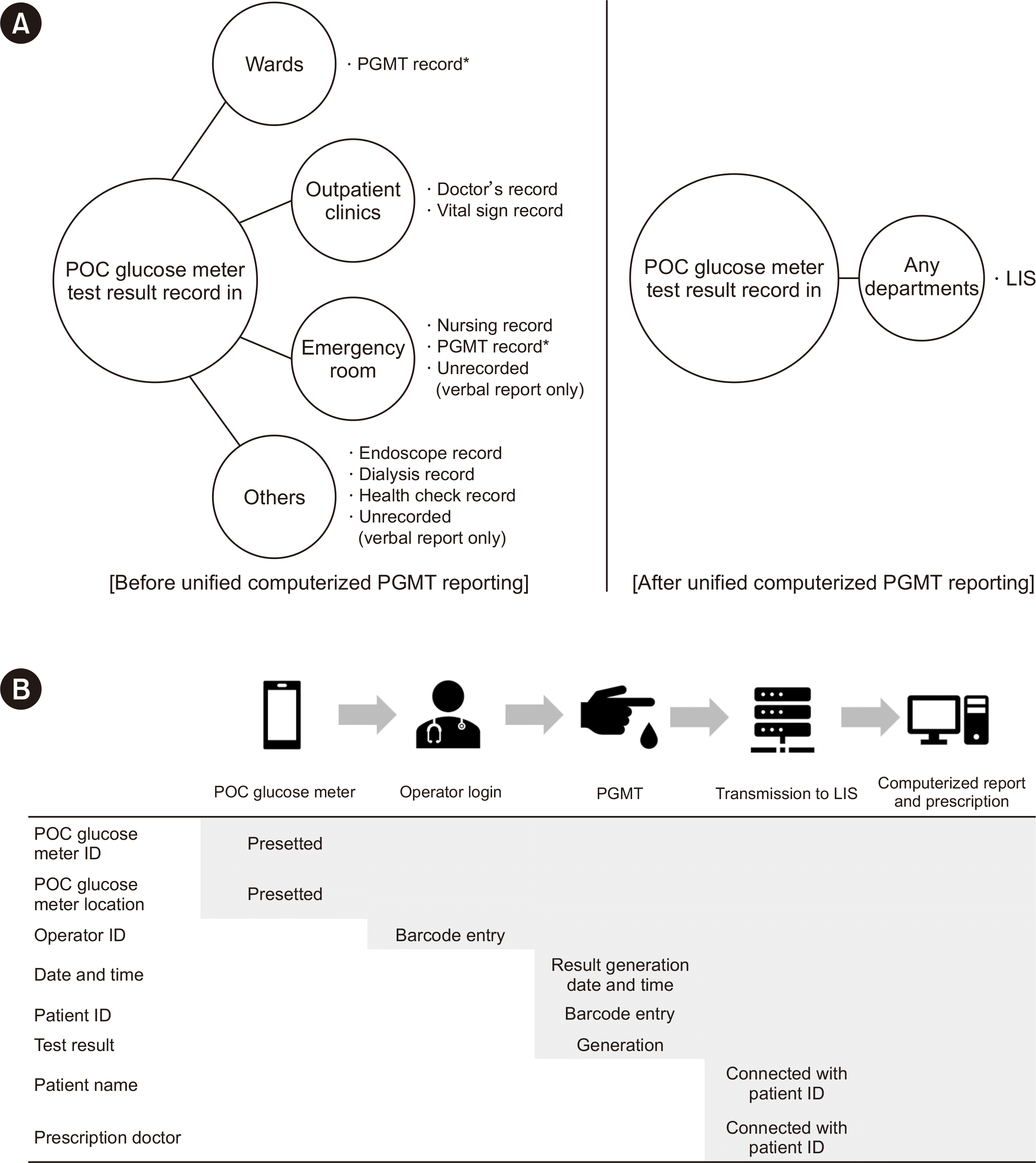
At Soonchunhyang University Bucheon Hospital (Bucheon, Korea), PGMT results had been processed through various procedures and methods, depending on the department where the patient is undergoing the test (Fig. 1A). We computerized and unified the reporting and prescription of PGMT (Fig. 1B). Barozen H Expert Plus (i-SENS, Seoul, Korea) was used as the POC glucose meter [6]. To evaluate the effect of unified computerized PGMT reporting, amount of test strip usage, accessible test results reported through the PGMT record or LIS, and number of requests (where “requests” refers to the charges for the tests) were reviewed before (January 1, 2019 to March 31, 2020) and after implementation (August 1, 2022 to December 31, 2022). The number of internal quality control (IQC) parameters before computerized reporting was approximated by multiplying the number of POC glucose meter by the count of QC materials and working days. All the statistical analyses were performed using Microsoft Excel 2019 (v1808; Microsoft Corp., Redmond, WA, USA). This study was approved by the Soonchunhyang University Bucheon Hospital Institutional Review Board (SCHBC 2023-03-005, noted April 2023), and the requirement for written informed consent was waived.
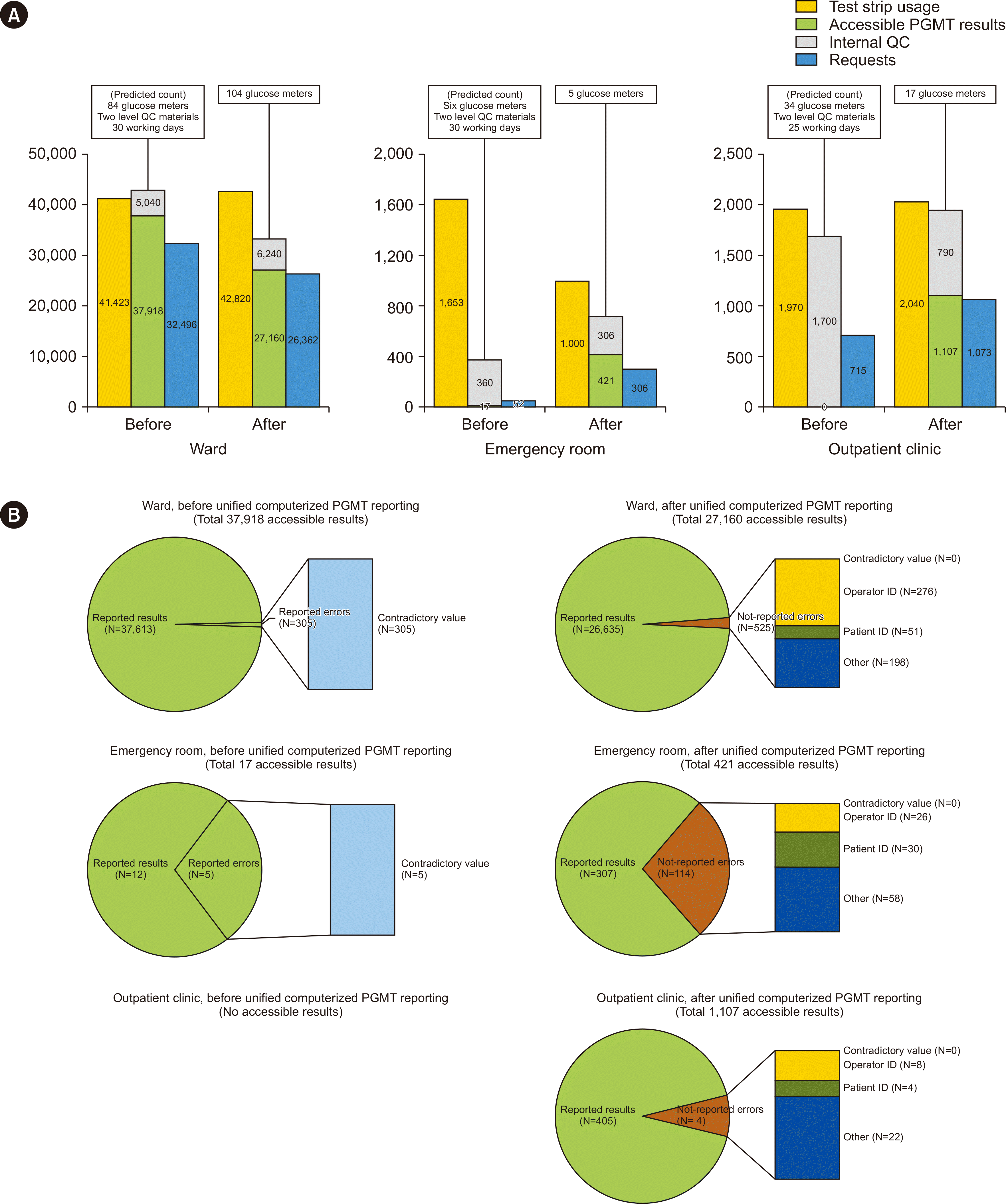
As shown in Fig. 2A, the sum of the number of accessible results and IQC parameters after computerized reporting decreased in wards but increased in emergency rooms and outpatient clinics. Before unification, we observed a discrepancy in the ward where the combined sum of the number of accessible results and the predicted IQC procedures surpassed the actual number of test strips. This inconsistency suggests the possibility that some IQC procedures, which should have been conducted in the ward, were not executed as predicted, resulting in a lower number of test strips actually used. During an interview conducted to determine why the sum of accessible results and IQC was 22%–27% less than the amount of test strip usage in the wards and emergency room after computerized reporting, the operators confessed that they improperly rejected some of the test results, despite having conducted the PGMT. This issue refers to the arbitrary rejection of results, leading to a lack of documented PGMT results. To address this problem, we plan to communicate this issue to department managers and provide targeted training to ensure that proper reporting procedures are followed. The simultaneous decrease in the number of accessible results and requests in wards may be interpreted as improved clinician access to the PGMT results, which, in turn, reduces duplicate testing [7]. The percentage of requests for accessible results increased from 85.7% to 97.1% in wards. In the emergency room and outpatient clinics, which had few accessible results before computerized reporting, the number of test strips used and number of requests were compared. Computerized reporting increased the request rate [8, 9]. As shown in Fig. 2B, contradictory values due to entry errors were eliminated after computerized reporting. Patient and operator ID errors observed after computerized reporting were flagged as abnormal and were not reported.
Unified computerized PGMT reporting ensures that all medical staff have access to updated information. This not only prevents duplicate testing but also enhances hospital operational efficiency by reducing the time and resources required to manage and analyze fragmented PGMT data [7, 10]. Computerized PGMT reporting also plays a crucial role in meeting the various requirements related to data reliability, such as minimizing result input errors, blocking the entry of incorrect operator IDs, and preventing omissions in IQC, of the Laboratory Medicine Foundation. The adoption of a unified computerized reporting model for various POC test results will contribute to the creation of standardized and reliable big datasets for future improvements in medical services.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the contributions of Jae Jun Lee, M.T., Jeong Gwon Kim, M.T., Yoon Jung Jin, R.N., Jae Min Lee, and Soojin Kim from Soonchunhyang University Bucheon Hospital and Hyeran Oh from the Soonchunhyang Medical Device Clinical Research Center.
Notes
AUTHOR CONTRIBUTIONS
Lee YK and Lee YW conceptualized the study. Lee YW was the project administrator. Choi S curated the data. Choi S, Choi SJ, and Kim JK investigated the parameters. Choi S designed the methodology. Lee YW validated the data. Choi S visualized the data and wrote the original draft. Choi D, Choi SJ, Kim JK, Lee YK, and Lee YW reviewed and edited the manuscript. All authors read and agreed to the published version of the manuscript.
REFERENCES
1. Kim S, Cho EJ, Jeong TD, Park HD, Yun YM, Lee K, et al. 2023; Proposed model for evaluating real-world laboratory results for big data research. Ann Lab Med. 43:104–7. DOI: 10.3343/alm.2023.43.1.104. PMID: 36045065. PMCID: PMC9467825.

2. Blatter TU, Witte H, Nakas CT, Leichtle AB. 2022; Big data in laboratory medicine-FAIR quality for AI? Diagnostics (Basel). 12:1923. DOI: 10.3390/diagnostics12081923. PMID: 36010273. PMCID: PMC9406962.

3. Choi S, Choi SJ, Jeon BR, Lee YW, Oh J, Lee YK. 2021; What we should consider in point of care blood glucose test; current quality management status of a single institution. Medicina (Kaunas). 57:238. DOI: 10.3390/medicina57030238. PMID: 33806620. PMCID: PMC8001912.

4. Khan AI, Khan M, Khan R. 2023; Artificial intelligence in point-of-care testing. Ann Lab Med. 43:401–7. DOI: 10.3343/alm.2023.43.5.401. PMID: 37080740. PMCID: PMC10151281.

5. Clarke SF, Foster JR. 2012; A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci. 69:83–93. DOI: 10.1080/09674845.2012.12002443. PMID: 22872934.

6. Choi S, Choi SJ, Kim JK, Lee YW, Lee YK. 2023; Real-world evidence of point-of-care glucometers: Enhanced passive surveillance and adverse event reporting status in Korea and the United States. Ann Lab Med. 43:515–9. DOI: 10.3343/alm.2023.43.5.515. PMID: 37080755. PMCID: PMC10151275.

7. Barglowski M. 2004; Beyond the glucose meter. Point Care. 3:26–9. DOI: 10.1097/00134384-200403000-00008.

8. Mays JA, Mathias PC. 2019; Measuring the rate of manual transcription error in outpatient point-of-care testing. J Am Med Inform Assoc. 26:269–72. DOI: 10.1093/jamia/ocy170. PMID: 30649499. PMCID: PMC6351970.

9. Carraro P, Plebani M. 2009; Post-analytical errors with portable glucose meters in the hospital setting. Clin Chim Acta. 404:65–7. DOI: 10.1016/j.cca.2009.03.013. PMID: 19298797.

10. Koproski J, Pretto Z, Poretsky L. 1997; Effects of an intervention by a diabetes team in hospitalized patients with diabetes. Diabetes Care. 20:1553–5. DOI: 10.2337/diacare.20.10.1553. PMID: 9314634.

Fig. 1
Schematic display of the PGMT result record. (A) PGMT results listed in various electronic health records are integrated into the LIS after unification. (B) After unification, reports and prescriptions were automatically created real time when the POC glucose meter ID, operator ID, patient ID, and PGMT results were transmitted to the LIS.
*Test results in the PGMT records were considered accessible.
Abbreviations: POC, point-of-care; PGMT, point-of-care glucose meter test; ID, identification; LIS, laboratory information system.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download