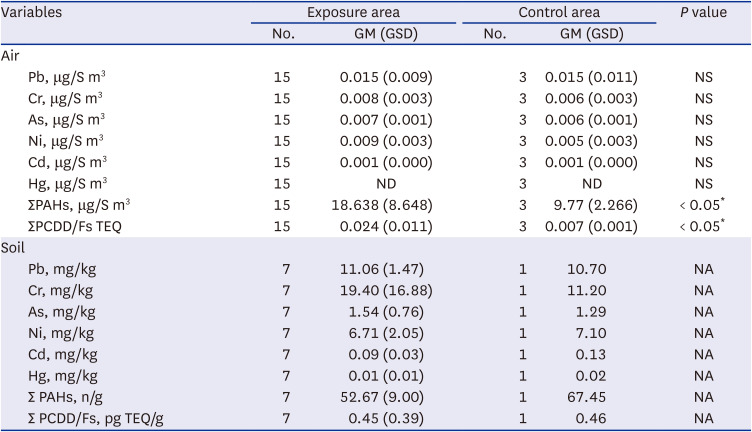
Hazardous substance concentration in the air and soil were measured in the area around the incinerator facility and in the control area. Air samples were collected for three days each in spring, summer, and autumn, and the average value was used for the analysis, whereas, the soil and agricultural products were measured once during summer. The sampling points were marked on the map (
Fig. 1). Harmful substances included six heavy metals, namely lead (Pb), chromium (Cr), arsenic (As), nickel (Ni), cadmium (Cd), mercury (Hg), and 16 PAHs (naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo [a]anthracene, Chrysene, Benzo[b]fluoranthene, benzo [k]fluoranthene, benzo [a]pyrene, indeno (1,2,3-cd)pyrene, dibenzo [a,h]anthracene, and benzo [ghi]perylene). Heavy metals were quantified using atomic absorption spectroscopy (AAS) after each pretreatment process, and PAHs were analyzed using gas chromatography/mass spectroscopy (GC/MS). All measurements were conducted according to the standard methods for the examination of environmental pollution by the Ministry of Environment, Korea. A total of 17 Polychlorinated dibenzo-p-dioxins and polychlorinated dibenzo-p-furans (PCDD/Fs) was measured in air and soil, and the detailed substances were as follows: 2,3,7,8-TeCDD, 1,2,3,7,8-PeCDD, 1,2,3,4,7,8-HxCDD, 1,2,3,6,7,8 -HxCDD, 1,2,3,7,8,9-HxCDD, 1,2,3,4,6,7,8-HpCDD, OCDD, 2,3,7,8-TeCDF, 1,2,3,7,8-PeCDF, 2,3,4,7,8-PeCDF, 1,2,3,4,7,8-HxCDF, 1,2,3,6,7,8-HxCDF, 2,3,4,6,7,8-HxCDF, 1,2,3,7,8,9-HxCDF, 1,2,3,4,6,7,8-HpCDF, 1,2,3,4,7,8,9-HpCDF, and OCDF. PCDDs/Fs were analyzed using high-resolution gas chromatography-high-resolution mass spectrometry (HRGC-HRMS). Instrumental analysis was conducted by Eurofins Scientific Korea and Donga University, who cooperated with our teams for quality assurance/quality control (QA/QC).





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download