This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The lack of well-established operational definitions is a major limitation of Helicobacter pylori eradication studies that use secondary databases. We aimed to develop and validate operational definitions related to H. pylori eradication therapy.
Methods
Operational definitions were developed by analyzing a nationwide H. pylori eradication registry and validated using real-world data from hospital medical records. The primary endpoint was the sensitivity of the operational definitions in identifying individuals who received H. pylori eradication therapy. The secondary endpoint was the sensitivity and specificity of the operational definition in identifying successful H. pylori eradication therapy.
Results
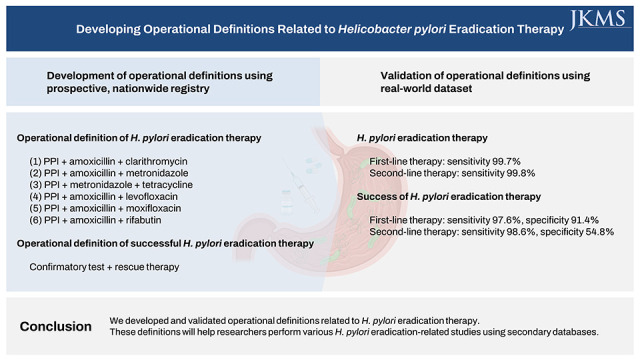
H. pylori eradication therapy was defined as a prescription for one of the following combinations: 1) proton pump inhibitor (PPI) + amoxicillin + clarithromycin, 2) PPI + amoxicillin + metronidazole, 3) PPI + metronidazole + tetracycline, 4) PPI + amoxicillin + levofloxacin, 5) PPI + amoxicillin + moxifloxacin, or 6) PPI + amoxicillin + rifabutin. In the validation set, the sensitivity of the operational definition for identifying individuals who received H. pylori eradication therapy was 99.7% and 99.8% for the first- and second-line therapies, respectively. Operational definition to determine success or failure of the H. pylori eradication therapy was developed based on a confirmatory test and the prescription of rescue therapy. The sensitivity and specificity of the operational definition for predicting successful eradication were 97.6% and 91.4%, respectively, in first-line therapy and 98.6% and 54.8%, respectively, in second-line therapy.
Conclusion
We developed and validated operational definitions related to H. pylori eradication therapy. These definitions will help researchers perform various H. pylori eradication-related studies using secondary databases.
Keywords: Helicobacter pylori, Eradication, Operational Definition
INTRODUCTION
Approximately 50% of the global population is infected with
Helicobacter pylori,
1 which causes various gastrointestinal diseases including peptic ulcer disease and gastric cancer.
23 Recently,
H. pylori infection has been associated with several diseases, including hematologic, cardiovascular, and neurological diseases, in addition to gastrointestinal diseases.
45 To determine the impact of
H. pylori eradication therapy on these diseases, population-based studies utilizing large-scale, nationwide, secondary databases have been performed.
678 The National Health Information Database of the Korean National Health Insurance Service (NHIS), which is a mandatory health insurance system that covers the entire Korean population, is one such database.
9 It has an extensive dataset of 1.3 trillion records, which includes sociodemographic status, diagnoses, operations, and prescriptions.
6910 It also includes follow-up data, spanning approximately 20 years.
611 Studies utilizing secondary databases may help clinicians better understand the clinical characteristics and treatment outcomes of rare diseases (e.g., metachronous lesions after gastric cancer treatment). Furthermore, they may help analyze diseases requiring long-term follow-up (e.g., the risk of gastric cancer in the general population). However, these studies may be limited by the lack of well-established operational definitions. In most studies,
H. pylori eradication was defined arbitrarily without validation.
678 Moreover, the success or failure of
H. pylori eradication therapy could not be determined because such results were unavailable in the secondary databases.
678 Because post-eradication persistence of
H. pylori infection is an important confounding variable in
H. pylori-related diseases, it is difficult to draw conclusions without knowing the outcome of the eradication therapy. Therefore, a definitive conclusion could not be reached by exploring the secondary databases alone.
To facilitate the use of secondary databases in studies and help reach a more definitive conclusion, we developed an operational definition of H. pylori eradication therapy. We also developed and validated operational definitions for predicting successful or failed treatment based on the prescriptions of eradication confirmatory tests and rescue therapy. These operational definitions will help researchers better analyze and interpret data from secondary databases.
METHODS
Study participants
Two databases of patients who underwent
H. pylori eradication therapy were used in this study. One was a nationwide registry (development set), which prospectively enrolled patients who received
H. pylori eradication therapy between 2010 and 2015 at 34 hospitals in South Korea.
12 The other database was a real-world dataset (validation set) of patients who received
H. pylori eradication therapy between 2018 and 2019 from four university-affiliated hospitals in South Korea (Chung-Ang University Hospital, Incheon St. Mary’s Hospital, Kangbuk Samsung Hospital, and Hanyang University Guri Hospital). The
H. pylori eradication regimen (i.e., active ingredients of medications) and type and results of the confirmatory test for
H. pylori eradication were reviewed.
Measurements and study endpoints
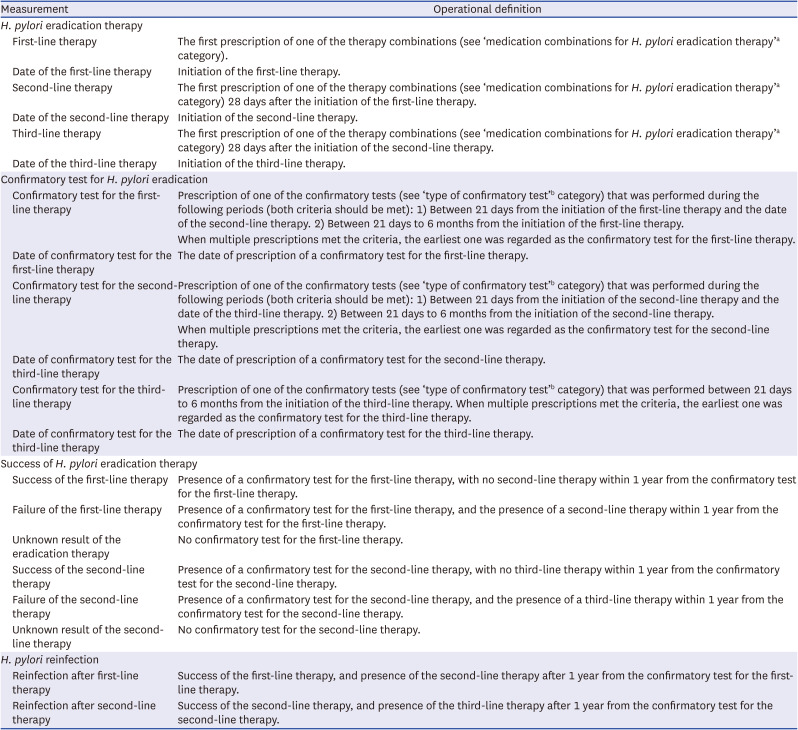
The operational definition of
H. pylori eradication therapy was developed based on the major regimens used in the nationwide registry and the current guidelines in Korea.
13 The intervals between the first- and second-line therapies and the second- and third-line therapies were evaluated to identify duplicate prescriptions or early changes in medications for any reason (loss of medications or adverse events). The interval between the prescription of eradication therapy and confirmatory test was also analyzed to ensure the validity of the confirmatory tests. The operational definition of success (or failure) of the eradication therapy was developed based on the presence of a confirmatory test or rescue therapy, the interval between eradication therapy and the confirmatory test, and that between the confirmatory test and rescue therapy initiation.
The primary endpoint was the sensitivity of the operational definition in identifying individuals who received H. pylori eradication therapy. The secondary endpoint was the sensitivity and specificity of the operational definition of a successful eradication therapy.
Statistical analysis
Descriptive statistics, including the number of participants and their proportions, were used to present the results. All statistical analyses were conducted using R (version 4.0.4; R Foundation for Statistical Computing, Vienna, Austria).
Ethics statement
This study was approved by the Institutional Review Board of study institutes and the requirement for informed consent was waived (Chung-Ang University Hospital, IRB No. 2110-014-19388; Incheon St. Mary's Hospital, IRB No. OC21RIDI0106; Kangbuk Samsung Hospital, IRB No. KBSMC 2021-09-026; Hanyang University Guri Hospital, IRB No. GURI 2021-09-009).
RESULTS
Identifying individuals who received H. pylori eradication therapy
We obtained and reviewed data of 6,740 and 1,572 patients, who received
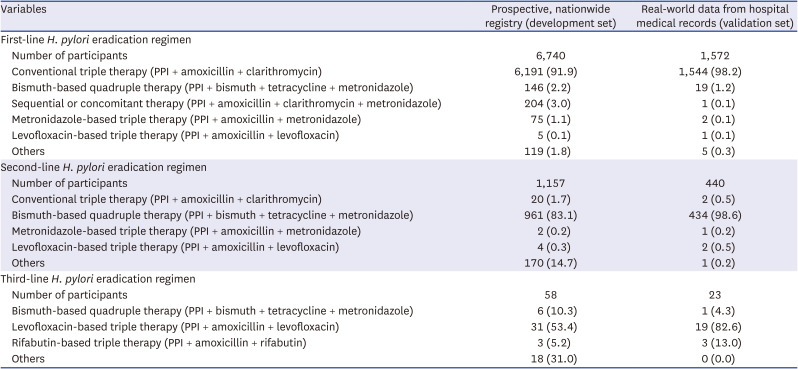
H. pylori eradication therapy, from the nationwide registry (development set) and real-world hospital medical records (validation set), respectively. The major
H. pylori eradication regimens utilized included conventional, metronidazole-based, levofloxacin-based, or rifabutin-based triple therapy and bismuth-based or non-bismuth (sequential or concomitant) quadruple therapy (
Table 1). Based on this,
H. pylori eradication therapy was defined as a prescription for one of the following combinations: 1) proton pump inhibitor (PPI) + amoxicillin + clarithromycin; 2) PPI + amoxicillin + metronidazole; 3) PPI + metronidazole + tetracycline; 4) PPI + amoxicillin + levofloxacin; 5) PPI + amoxicillin + moxifloxacin; and 6) PPI + amoxicillin + rifabutin (
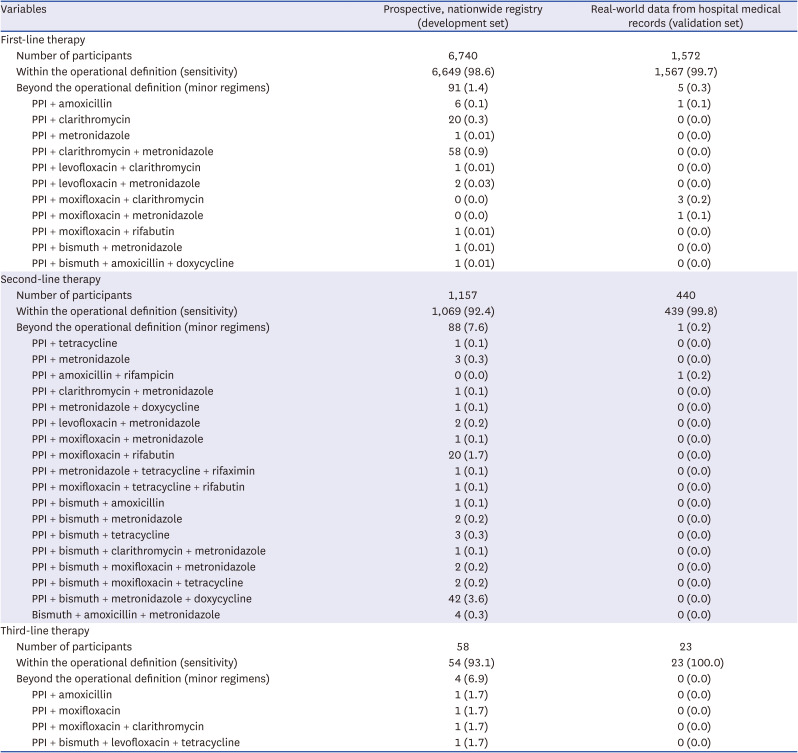
Table 2). All the major regimens were screened for these combinations. Using the operational definition of
H. pylori eradication therapy, 98.6% and 99.7% of the patients who received first-line
H. pylori eradication therapy were identified in the development and validation sets, respectively (
Table 3). Additionally, 92.4% and 99.8% of the patients who received second-line
H. pylori eradication therapy were identified in the development and validation sets, respectively.
Table 1
H. pylori eradication regimens included in the study
|
Variables |
Prospective, nationwide registry (development set) |
Real-world data from hospital medical records (validation set) |
|
First-line H. pylori eradication regimen |
|
|
|
Number of participants |
6,740 |
1,572 |
|
Conventional triple therapy (PPI + amoxicillin + clarithromycin) |
6,191 (91.9) |
1,544 (98.2) |
|
Bismuth-based quadruple therapy (PPI + bismuth + tetracycline + metronidazole) |
146 (2.2) |
19 (1.2) |
|
Sequential or concomitant therapy (PPI + amoxicillin + clarithromycin + metronidazole) |
204 (3.0) |
1 (0.1) |
|
Metronidazole-based triple therapy (PPI + amoxicillin + metronidazole) |
75 (1.1) |
2 (0.1) |
|
Levofloxacin-based triple therapy (PPI + amoxicillin + levofloxacin) |
5 (0.1) |
1 (0.1) |
|
Others |
119 (1.8) |
5 (0.3) |
|
Second-line H. pylori eradication regimen |
|
|
|
Number of participants |
1,157 |
440 |
|
Conventional triple therapy (PPI + amoxicillin + clarithromycin) |
20 (1.7) |
2 (0.5) |
|
Bismuth-based quadruple therapy (PPI + bismuth + tetracycline + metronidazole) |
961 (83.1) |
434 (98.6) |
|
Metronidazole-based triple therapy (PPI + amoxicillin + metronidazole) |
2 (0.2) |
1 (0.2) |
|
Levofloxacin-based triple therapy (PPI + amoxicillin + levofloxacin) |
4 (0.3) |
2 (0.5) |
|
Others |
170 (14.7) |
1 (0.2) |
|
Third-line H. pylori eradication regimen |
|
|
|
|
Number of participants |
58 |
23 |
|
Bismuth-based quadruple therapy (PPI + bismuth + tetracycline + metronidazole) |
6 (10.3) |
1 (4.3) |
|
Levofloxacin-based triple therapy (PPI + amoxicillin + levofloxacin) |
31 (53.4) |
19 (82.6) |
|
Rifabutin-based triple therapy (PPI + amoxicillin + rifabutin) |
3 (5.2) |
3 (13.0) |
|
Others |
18 (31.0) |
0 (0.0) |
Table 2
Operational definition of H. pylori eradication therapy and its consequences
|
Measurement |
Operational definition |
|
H. pylori eradication therapy |
|
|
First-line therapy |
The first prescription of one of the therapy combinations (see ‘medication combinations for H. pylori eradication therapy’a category). |
|
Date of the first-line therapy |
Initiation of the first-line therapy. |
|
Second-line therapy |
The first prescription of one of the therapy combinations (see ‘medication combinations for H. pylori eradication therapy’a category) 28 days after the initiation of the first-line therapy. |
|
Date of the second-line therapy |
Initiation of the second-line therapy. |
|
Third-line therapy |
The first prescription of one of the therapy combinations (see ‘medication combinations for H. pylori eradication therapy’a category) 28 days after the initiation of the second-line therapy. |
|
Date of the third-line therapy |
Initiation of the third-line therapy. |
|
Confirmatory test for H. pylori eradication |
|
|
Confirmatory test for the first-line therapy |
Prescription of one of the confirmatory tests (see ‘type of confirmatory test’b category) that was performed during the following periods (both criteria should be met): 1) Between 21 days from the initiation of the first-line therapy and the date of the second-line therapy. 2) Between 21 days to 6 months from the initiation of the first-line therapy. |
|
When multiple prescriptions met the criteria, the earliest one was regarded as the confirmatory test for the first-line therapy. |
|
Date of confirmatory test for the first-line therapy |
The date of prescription of a confirmatory test for the first-line therapy. |
|
Confirmatory test for the second-line therapy |
Prescription of one of the confirmatory tests (see ‘type of confirmatory test’b category) that was performed during the following periods (both criteria should be met): 1) Between 21 days from the initiation of the second-line therapy and the date of the third-line therapy. 2) Between 21 days to 6 months from the initiation of the second-line therapy. |
|
When multiple prescriptions met the criteria, the earliest one was regarded as the confirmatory test for the second-line therapy. |
|
Date of confirmatory test for the third-line therapy |
The date of prescription of a confirmatory test for the second-line therapy. |
|
Confirmatory test for the third-line therapy |
Prescription of one of the confirmatory tests (see ‘type of confirmatory test’b category) that was performed between 21 days to 6 months from the initiation of the third-line therapy. When multiple prescriptions met the criteria, the earliest one was regarded as the confirmatory test for the third-line therapy. |
|
Date of confirmatory test for the third-line therapy |
The date of prescription of a confirmatory test for the third-line therapy. |
|
Success of H. pylori eradication therapy |
|
|
Success of the first-line therapy |
Presence of a confirmatory test for the first-line therapy, with no second-line therapy within 1 year from the confirmatory test for the first-line therapy. |
|
Failure of the first-line therapy |
Presence of a confirmatory test for the first-line therapy, and the presence of a second-line therapy within 1 year from the confirmatory test for the first-line therapy. |
|
Unknown result of the eradication therapy |
No confirmatory test for the first-line therapy. |
|
Success of the second-line therapy |
Presence of a confirmatory test for the second-line therapy, with no third-line therapy within 1 year from the confirmatory test for the second-line therapy. |
|
Failure of the second-line therapy |
Presence of a confirmatory test for the second-line therapy, and the presence of a third-line therapy within 1 year from the confirmatory test for the second-line therapy. |
|
Unknown result of the second-line therapy |
No confirmatory test for the second-line therapy. |
|
H. pylori reinfection |
|
|
Reinfection after first-line therapy |
Success of the first-line therapy, and presence of the second-line therapy after 1 year from the confirmatory test for the first-line therapy. |
|
Reinfection after second-line therapy |
Success of the second-line therapy, and presence of the third-line therapy after 1 year from the confirmatory test for the second-line therapy. |
Table 3
Sensitivity of the operational definition for identifying individuals who received H. pylori eradication therapy
|
Variables |
Prospective, nationwide registry (development set) |
Real-world data from hospital medical records (validation set) |
|
First-line therapy |
|
|
|
Number of participants |
6,740 |
1,572 |
|
Within the operational definition (sensitivity) |
6,649 (98.6) |
1,567 (99.7) |
|
Beyond the operational definition (minor regimens) |
91 (1.4) |
5 (0.3) |
|
|
PPI + amoxicillin |
6 (0.1) |
1 (0.1) |
|
|
PPI + clarithromycin |
20 (0.3) |
0 (0.0) |
|
|
PPI + metronidazole |
1 (0.01) |
0 (0.0) |
|
|
PPI + clarithromycin + metronidazole |
58 (0.9) |
0 (0.0) |
|
|
PPI + levofloxacin + clarithromycin |
1 (0.01) |
0 (0.0) |
|
|
PPI + levofloxacin + metronidazole |
2 (0.03) |
0 (0.0) |
|
|
PPI + moxifloxacin + clarithromycin |
0 (0.0) |
3 (0.2) |
|
|
PPI + moxifloxacin + metronidazole |
0 (0.0) |
1 (0.1) |
|
|
PPI + moxifloxacin + rifabutin |
1 (0.01) |
0 (0.0) |
|
|
PPI + bismuth + metronidazole |
1 (0.01) |
0 (0.0) |
|
|
PPI + bismuth + amoxicillin + doxycycline |
1 (0.01) |
0 (0.0) |
|
Second-line therapy |
|
|
|
Number of participants |
1,157 |
440 |
|
Within the operational definition (sensitivity) |
1,069 (92.4) |
439 (99.8) |
|
Beyond the operational definition (minor regimens) |
88 (7.6) |
1 (0.2) |
|
|
PPI + tetracycline |
1 (0.1) |
0 (0.0) |
|
|
PPI + metronidazole |
3 (0.3) |
0 (0.0) |
|
|
PPI + amoxicillin + rifampicin |
0 (0.0) |
1 (0.2) |
|
|
PPI + clarithromycin + metronidazole |
1 (0.1) |
0 (0.0) |
|
|
PPI + metronidazole + doxycycline |
1 (0.1) |
0 (0.0) |
|
|
PPI + levofloxacin + metronidazole |
2 (0.2) |
0 (0.0) |
|
|
PPI + moxifloxacin + metronidazole |
1 (0.1) |
0 (0.0) |
|
|
PPI + moxifloxacin + rifabutin |
20 (1.7) |
0 (0.0) |
|
|
PPI + metronidazole + tetracycline + rifaximin |
1 (0.1) |
0 (0.0) |
|
|
PPI + moxifloxacin + tetracycline + rifabutin |
1 (0.1) |
0 (0.0) |
|
|
PPI + bismuth + amoxicillin |
1 (0.1) |
0 (0.0) |
|
|
PPI + bismuth + metronidazole |
2 (0.2) |
0 (0.0) |
|
|
PPI + bismuth + tetracycline |
3 (0.3) |
0 (0.0) |
|
|
PPI + bismuth + clarithromycin + metronidazole |
1 (0.1) |
0 (0.0) |
|
|
PPI + bismuth + moxifloxacin + metronidazole |
2 (0.2) |
0 (0.0) |
|
|
PPI + bismuth + moxifloxacin + tetracycline |
2 (0.2) |
0 (0.0) |
|
|
PPI + bismuth + metronidazole + doxycycline |
42 (3.6) |
0 (0.0) |
|
|
Bismuth + amoxicillin + metronidazole |
4 (0.3) |
0 (0.0) |
|
Third-line therapy |
|
|
|
Number of participants |
58 |
23 |
|
Within the operational definition (sensitivity) |
54 (93.1) |
23 (100.0) |
|
Beyond the operational definition (minor regimens) |
4 (6.9) |
0 (0.0) |
|
|
PPI + amoxicillin |
1 (1.7) |
0 (0.0) |
|
|
PPI + moxifloxacin |
1 (1.7) |
0 (0.0) |
|
|
PPI + moxifloxacin + clarithromycin |
1 (1.7) |
0 (0.0) |
|
|
PPI + bismuth + levofloxacin + tetracycline |
1 (1.7) |
0 (0.0) |
Treatment duration, interval, and confirmatory tests
The durations of the first-, second-, and third-line eradication therapies are shown in
Supplementary Fig. 1.
H. pylori eradication therapies were typically administered for 7–14 days.
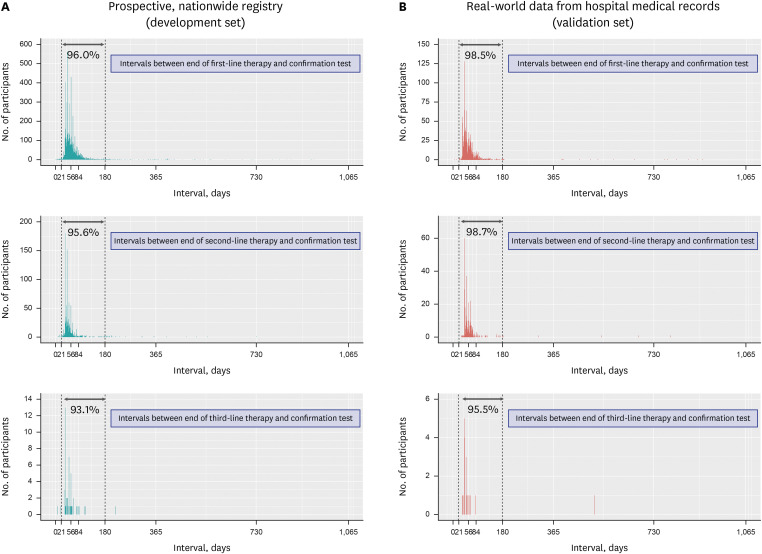
Fig. 1 shows the treatment intervals between
the H. pylori eradication therapies, which was ≥ 28 days in > 99% of patients. Only a few patients had a treatment interval of < 28 days, where the initial treatment was stopped early owing to adverse events; thereafter, rescue therapy was administered. The confirmatory test was employed in 98.6% and 87.0% of the patients in the development and validation sets, respectively (
Supplementary Table 1). In most patients, a confirmatory test was performed 21 days to six months after the initiation of
H. pylori eradication therapy (
Fig. 2). Based on these findings, we defined confirmatory tests as the prescription of the confirmatory tests during the following periods: 1) between 21 days from the initiation of the eradication therapy and the date of rescue therapy, and 2) between 21 days to six months from the initiation of eradication therapy (both criteria should be met) (
Table 2).
Fig. 1
Treatment intervals between H. pylori eradication therapies in the nationwide registry (A) and real-world data from hospital medical records (B). Gray arrows and percentages indicate the proportion of patients with treatment intervals ≥ 28 days.
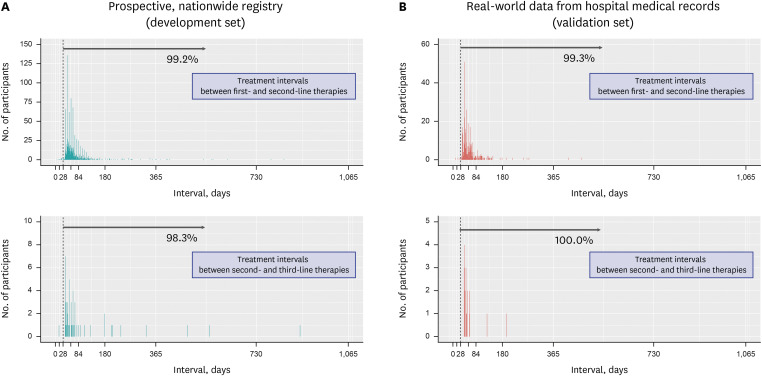
Fig. 2
Intervals between completion of the H. pylori eradication therapy and performance of the eradication confirmatory test in the prospective, nationwide registry (A) and real-world data from hospital medical records (B). Gray arrows and percentages indicate the proportion of patients who underwent testing for confirmation of H. pylori eradication 21 days to 6 months after initiation of eradication therapy.
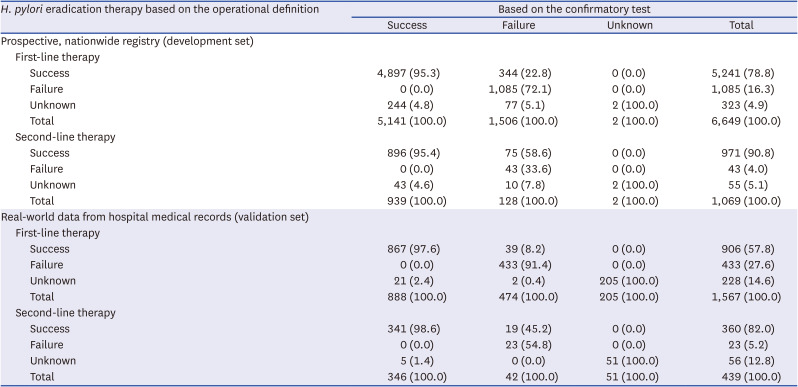
Determining the success or failure of H. pylori eradication therapy
Subsequently, we tested and validated the diagnostic performance of the operational definition in determining the success or failure of
H. pylori eradication therapy (
Table 4). In the development set, the sensitivity and specificity of the operational definition for predicting successful eradication were 95.3% and 72.1%, respectively, for first-line therapy and 95.4% and 33.6%, respectively, for second-line therapy. In the validation set, the sensitivity and specificity were 97.6% and 91.4%, respectively, for first-line therapy and 98.6% and 54.8%, respectively, for second-line therapy.
Table 4
Performance of the operational definition in determining successful or failed H. pylori eradication therapy
|
H. pylori eradication therapy based on the operational definition |
Based on the confirmatory test |
|
Success |
Failure |
Unknown |
Total |
|
Prospective, nationwide registry (development set) |
|
|
|
|
|
First-line therapy |
|
|
|
|
|
|
Success |
4,897 (95.3) |
344 (22.8) |
0 (0.0) |
5,241 (78.8) |
|
|
Failure |
0 (0.0) |
1,085 (72.1) |
0 (0.0) |
1,085 (16.3) |
|
|
Unknown |
244 (4.8) |
77 (5.1) |
2 (100.0) |
323 (4.9) |
|
|
Total |
5,141 (100.0) |
1,506 (100.0) |
2 (100.0) |
6,649 (100.0) |
|
Second-line therapy |
|
|
|
|
|
|
Success |
896 (95.4) |
75 (58.6) |
0 (0.0) |
971 (90.8) |
|
|
Failure |
0 (0.0) |
43 (33.6) |
0 (0.0) |
43 (4.0) |
|
|
Unknown |
43 (4.6) |
10 (7.8) |
2 (100.0) |
55 (5.1) |
|
|
Total |
939 (100.0) |
128 (100.0) |
2 (100.0) |
1,069 (100.0) |
|
Real-world data from hospital medical records (validation set) |
|
|
|
|
|
First-line therapy |
|
|
|
|
|
|
Success |
867 (97.6) |
39 (8.2) |
0 (0.0) |
906 (57.8) |
|
|
Failure |
0 (0.0) |
433 (91.4) |
0 (0.0) |
433 (27.6) |
|
|
Unknown |
21 (2.4) |
2 (0.4) |
205 (100.0) |
228 (14.6) |
|
|
Total |
888 (100.0) |
474 (100.0) |
205 (100.0) |
1,567 (100.0) |
|
Second-line therapy |
|
|
|
|
|
|
Success |
341 (98.6) |
19 (45.2) |
0 (0.0) |
360 (82.0) |
|
|
Failure |
0 (0.0) |
23 (54.8) |
0 (0.0) |
23 (5.2) |
|
|
Unknown |
5 (1.4) |
0 (0.0) |
51 (100.0) |
56 (12.8) |
|
|
Total |
346 (100.0) |
42 (100.0) |
51 (100.0) |
439 (100.0) |
Additionally, the criteria for
H. pylori reinfection was set as the success of the initial eradication therapy followed by the administration of additional therapy one year after the initial confirmatory test (
Table 2). However, the operational definition of reinfection could not be validated in this study because no participant with reinfection of
H. pylori was identified in the validation set.
Sensitivity analysis
We evaluated the
H. pylori eradication regimen, sensitivity of the operational definition for identifying individuals who received eradication therapy, and performance of the operational definition in determining the success or failure of eradication therapy according to the hospital in the validation set (
Supplementary Tables 2,
3,
4). Although the sample size of each hospital was small, the major results were similar to the overall results in the validation set.
DISCUSSION
This is the first study to assess the operational definition of
H. pylori eradication therapy. We developed an operational definition based on the major regimens utilized in the prospective nationwide registry established in 2010–2015. Minor regimens, including dual therapy (e.g., PPI + amoxicillin, PPI + clarithromycin, or PPI + metronidazole) and quinolone-containing clarithromycin-based therapy (e.g., PPI + levofloxacin + clarithromycin or PPI + moxifloxacin + clarithromycin), were excluded from the operational definition. Dual therapy is not popular in Korea because of its low eradication rate.
1415 Quinolone-based therapies are not favored, except for third-line treatment (e.g., PPI + amoxicillin + quinolone), because it is also used to treat tuberculosis in Korea, which is highly prevalent. Although some regimens were not included in the operational definition, its sensitivity to detect
H. pylori eradication therapy was high, even in the validation set. This finding suggests that most regimens used in real-world settings can be predicted. The high sensitivity may be attributed to the dissemination of common guidelines and the fact that antibiotics not covered by the NHIS were infrequently prescribed in the real world. All regimens recommended in the Korean guidelines, including triple, sequential, concomitant, and bismuth-based quadruple therapies, were included in the operational definition.
13
In the present study, second- or third-line therapies was defined as the prescription of one of the H. pylori eradication regimens 28 days after the previous eradication therapy. The time interval of 28 days was selected to exclude duplicate prescriptions for lost medications or early changes in the eradication regimen. In our analysis, approximately 99% of the participants received rescue therapy 28 days after their initial therapy. Given that the performance of confirmatory tests for H. pylori eradication and issuance of rescue therapy prescriptions typically occur at least 3–4 weeks after treatment, the operational definition was clinically relevant and reliable.
We also set the time interval between eradication therapy and the confirmatory test as 21 days to six months, because most participants obtained a confirmatory test during this time period. Although very few participants were subjected to confirmatory tests six months after the treatment, an interval of more than six months was not considered valid as the minimum follow-up duration was six months. The time interval was limited to six months because it may be difficult to distinguish between treatment failure and reinfection if tests are conducted after a long time.
Finally, we developed an operational definition to determine success or failure of the H. pylori eradication therapy based on a confirmatory test and the prescription of rescue therapy. In the validation set, the sensitivity and specificity for determining successful H. pylori eradication with first-line treatment were sufficiently high. Thus, the results of the first-line H. pylori eradication therapy could be determined without knowing the exact results of the confirmatory test in the secondary databases. However, the specificity for determining successful H. pylori eradication therapy after second-line treatment was relatively low. This is because administration of third-line therapy is uncommon in the real-world setting. The operational definition may mistakenly classify individuals in whom second-line therapy failed and who were not administered third-line therapy as having received successful second-line therapy. Therefore, caution should be exercised when interpreting the results of the second-line therapy.
Although our study is the first to develop and validate an operational definition of H. pylori eradication therapy, it has several limitations. First, the operational definition was developed in Korea considering the local clinical settings and preferences, including the major eradication regimens. If the operational definition is applied to other countries or regions, the preferred H. pylori eradication regimens of that country/region should be considered. Second, the institutions included in the development and validation sets overlapped, indicating that the development and validation sets may depend on each other. However, the institutions included in the validation set accounted for only a small proportion of the nationwide registry, and the study periods for the development and validation sets were distinctly separated. Moreover, the impact of the bias caused by interdependency between the development and validation sets may have been minimal. Third, the operational definition of H. pylori reinfection was not validated because of the lack of participants with reinfection in the validation set. Fourth, we did not include potassium-competitive acid blockers (P-CABs) in our operational definitions. Although tegoprazan, a P-CAB, has been approved for H. pylori eradication therapy in Korea in 2021, our study included only patients who received H. pylori eradication therapy in 2019 or before. We plan to extend our operational definitions to include patients who received P-CAB-based H. pylori eradication therapy. Although the operational definition for reinfection seems to be clinically relevant, long-term follow-up data are required to validate them. Fifth, unknown treatment results could not be ignored in the validation set, as some patients could have been lost to follow-up or may not have undergone confirmatory tests in real-world practice. Operational definitions do not provide information on the success of eradication therapy in such patients. Finally, the practical use of operational definitions in secondary databases, such as the NHIS claim database, was not tested. We plan to apply the operational definition to perform various subsequent clinical studies. A modified version of the operational definition may be derived for subsequent studies.
Despite these limitations, our study provides valid and applicable operational definitions that can be used for H. pylori-related studies utilizing a secondary database. Using prescriptions of major H. pylori eradication regimens, we developed an operational definition for identifying individuals who received H. pylori eradication therapy. Additionally, the operational definition of a successful or failed eradication therapy was based on the presence of a confirmatory test and rescue therapy prescription. Our operational definitions may be useful for various studies that utilize secondary databases.










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download