INTRODUCTION
Several antiviral agents have been used against coronavirus disease 2019 (COVID-19). Nirmatrelvir is a potent inhibitor that binds to the main protease of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and affects viral replication.
1 Nirmatrelvir was first authorized as an oral antiviral agent by the United States Food and Drug Administration in December 2021 for emergency use for treating mild-to-moderate COVID-19 in patients at high risk of progression to severe disease.
2 The EPIC-HR trial—a randomized controlled trial on high-risk patients—demonstrated that nirmatrelvir reduced the progression to severe COVID-19 by about 89% in unvaccinated adult patients who were symptomatic for 5 days or less.
3 Given that previously authorized antivirals or monoclonal antibody agents against COVID-19 were limited to injectable drugs, a potent oral antiviral agent for non-hospitalized patients with the mild-to-moderate disease is expected to improve COVID-19 outcomes.
4 Notably, the EPIC-HR trial was conducted only on unvaccinated SARS-CoV-2-infected patients during a period dominated by the B.1.617.2 (delta) variant.
However, given that more than 85% of adults aged ≥ 60 years received three doses of the COVID-19 vaccine as of January 31, 2022, in South Korea
5 and that other predominant variants have emerged since the delta variant, the real-world performance on the effectiveness of nirmatrelvir-ritonavir needs to be elucidated in South Korea. Consequently, we aimed to investigate the effectiveness of nirmatrelvir-ritonavir in preventing progression to severe disease in high-risk patients with mild-to-moderate COVID-19, bearing in mind the current context of the predominance of the omicron variant and high levels of vaccine coverage in South Korea.
RESULTS
Baseline characteristics of the participants
A total of 2,782 patients aged 40 years and older were admitted to the four study hospitals during the study period (
Fig. 1). After excluding 2,148 patients according to the specified inclusion and exclusion criteria, 634 patients were finally enrolled, with 258 and 376 patients in the treatment and control groups, respectively. Treatment group patients, when compared to those of the control group, were found to be older (69.4 vs. 66.2 years, respectively), had a higher proportion of men (47.7% vs. 40.2%, respectively), and had a higher proportion of comorbidities (96.5% vs. 90.6%, respectively) (
Table 1,
Supplementary Table 1). In particular, treatment group patients had a higher proportion of solid tumors (23.2% vs. 15.1%, respectively,
P = 0.010) and greater immunosuppressant use (9.3% vs. 3.1%, respectively,
P = 0.001) than those of the control group. In line with the indication for nirmatrelvir-ritonavir, the treatment group presented more frequently with pneumonia on chest radiography than the control group (16.2% vs. 7.8%, respectively,
P = 0.001). Further, the duration from symptom onset to admission was shorter in the treatment group than in the control group (1.9 days vs. 2.4 days, respectively,
P < 0.001). In the treatment group, the mean time from admission to the first dose of nirmatrelvir-ritonavir was 2.0 days. COVID-19 vaccination status was similar between the treatment and control groups (76.7% and 74.2%, respectively).
Fig. 1
Flowchart for selection of study participants.
SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
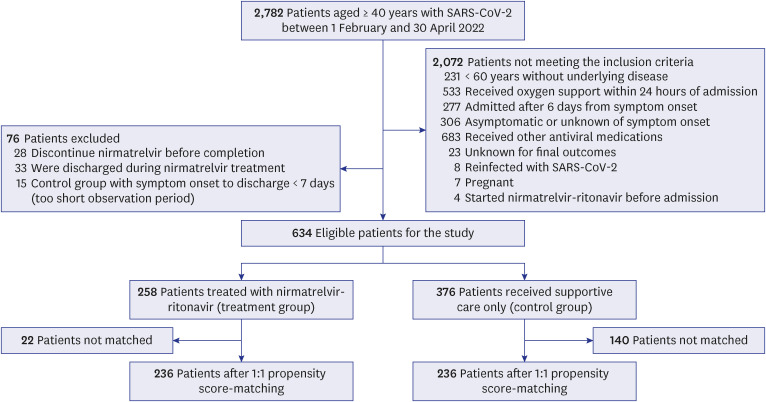

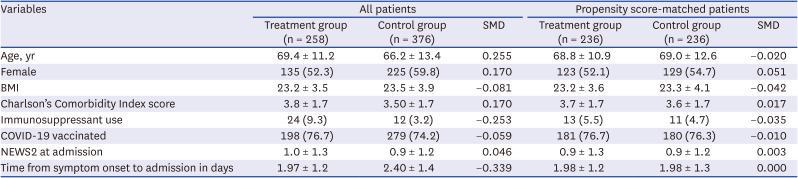
Table 1
Characteristics of COVID-19 patients before and after propensity score-matching
|
Variables |
All patients |
Propensity score-matched patients |
|
Treatment group (n = 258) |
Control group (n = 376) |
SMD |
Treatment group (n = 236) |
Control group (n = 236) |
SMD |
|
Age, yr |
69.4 ± 11.2 |
66.2 ± 13.4 |
0.255 |
68.8 ± 10.9 |
69.0 ± 12.6 |
−0.020 |
|
Female |
135 (52.3) |
225 (59.8) |
0.170 |
123 (52.1) |
129 (54.7) |
0.051 |
|
BMI |
23.2 ± 3.5 |
23.5 ± 3.9 |
−0.081 |
23.2 ± 3.6 |
23.3 ± 4.1 |
−0.042 |
|
Charlson’s Comorbidity Index score |
3.8 ± 1.7 |
3.50 ± 1.7 |
0.170 |
3.7 ± 1.7 |
3.6 ± 1.7 |
0.017 |
|
Immunosuppressant use |
24 (9.3) |
12 (3.2) |
−0.253 |
13 (5.5) |
11 (4.7) |
−0.035 |
|
COVID-19 vaccinated |
198 (76.7) |
279 (74.2) |
−0.059 |
181 (76.7) |
180 (76.3) |
−0.010 |
|
NEWS2 at admission |
1.0 ± 1.3 |
0.9 ± 1.2 |
0.046 |
0.9 ± 1.3 |
0.9 ± 1.2 |
0.003 |
|
Time from symptom onset to admission in days |
1.97 ± 1.2 |
2.40 ± 1.4 |
−0.339 |
1.98 ± 1.2 |
1.98 ± 1.3 |
0.000 |

Subsequently, 236 patients were matched between the treatment and control groups for analysis. The demographic and clinical characteristics of the two groups before and after PS-matching are presented in
Table 1. After matching, the treatment and control groups were similar in terms of age (68.8 vs. 69.0 years, respectively), sex (percentage of women: 52.1% vs. 54.7%, respectively), presence of comorbidities, vaccination status, and NEWS2 at admission. Standardized differences were less than 0.1 for all measures.
Primary outcome: oxygen support-free survival
Among the total 472 matched patients, 2 (0.8%) patients in the treatment group and 22 (9.3%) patients in the control group needed new oxygen support, with a median time from admission to initial oxygen support of 6 days (interquartile range [IQR], 5–7) in the treatment group and 6 days (IQR, 5–7) in the control group. The Kaplan–Meier survival curves for oxygen support-free survival (
Fig. 2) showed that there was a significant difference in the time from admission to oxygen support or discharge between the two groups through a follow-up of 11 days (
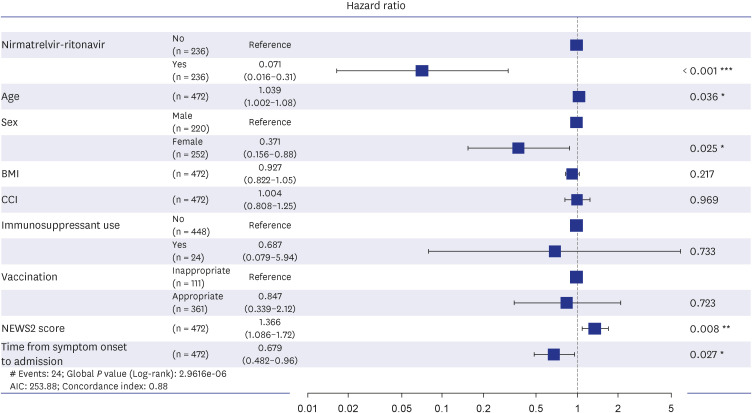
P < 0.001). The multivariate Cox regression model showed that nirmatrelvir-ritonavir significantly reduced the need for oxygen therapy compared to that of the control group (adjusted hazard ratio [aHR], 0.07; 95% CI, 0.01–0.31) (
Fig. 3). Female sex (aHR, 0.37; 95% CI, 0.15–0.88;
P = 0.025) and duration from symptom onset to admission (aHR, 0.679; 95% CI, 0.48–0.96;
P = 0.027) were also associated with less need for oxygen support. However, high NEWS2 (aHR, 1.36; 95% CI, 1.08–1.72;
P = 0.008) and old age (aHR, 1.039; 95% CI, 1.00–1.08;
P = 0.036) were associated with disease progression to requiring oxygen support. BMI, vaccination status, and underlying diseases (CCI) did not show a significant association with oxygen support.
Fig. 2
Oxygen support-free survival analysis in the propensity score–matched cohort. Kaplan–Meier survival curves of oxygen-free survival with a follow-up duration of 11 days from the hospital admission. Patients who were discharged without oxygen therapy were censored at the discharge date or on hospital day 12, whichever came first. The nirmatrelvir-ritonavir treatment group showed significantly longer oxygen-free survival (P < 0.001) than the control group.
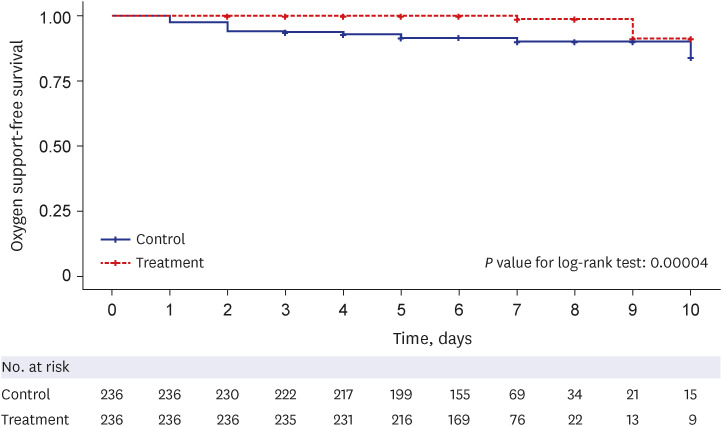

Fig. 3
The forest plot of multivariate Cox proportional analysis of oxygen therapy with nirmatrelvir-ritonavir treatment. The following variables were included in the model: age, sex, BMI, CCI score, immunosuppressant use, coronavirus disease 2019 vaccination status, NEWS2, and days from symptom onset to admission. Inappropriate vaccination indicates patients who were never vaccinated or received only one dose of vaccine or a second dose more than 180 days prior to enrollment date. Appropriate vaccination indicates patients who received a third dose of vaccine within 8 days or a second dose between 8 and 180 days before the enrollment date.
BMI = body mass index, CCI = Charlson Comorbidity Index, NEWS2 = National Early Warning Score-2.
*P < 0.05, **P < 0.01, ***P < 0.001.

Composite outcomes for the progression to severe disease or mortality
Regarding the composite outcomes for progression to severe disease (facial mask, high-flow nasal cannula, and invasive mechanical ventilator) and mortality, 1 (0.4%) patient in the matched treatment group and 4 (1.7%) patients in the matched control group progressed to severe diseases beyond nasal cannula; all 5 of them died. Thus, the all-cause in-hospital mortality was 0.4% in the treatment group and 1.7% in the control group, and the difference between the two groups was not statistically significant. According to the multivariate Cox regression model, no factor was significantly associated with the composite outcome (
Table 2).
Table 2
Multivariable Cox regression analysis of the requirement of oxygen support, severe COVID-19, and mortality
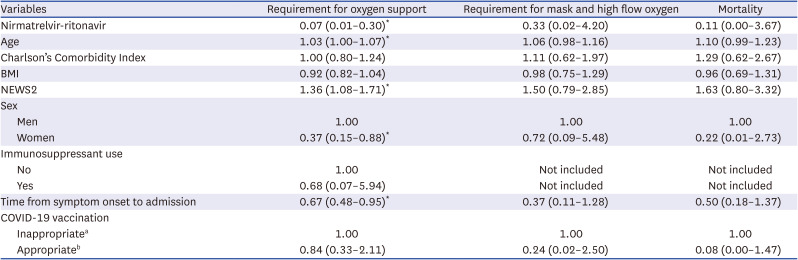
|
Variables |
Requirement for oxygen support |
Requirement for mask and high flow oxygen |
Mortality |
|
Nirmatrelvir-ritonavir |
0.07 (0.01–0.30)*
|
0.33 (0.02–4.20) |
0.11 (0.00–3.67) |
|
Age |
1.03 (1.00–1.07)*
|
1.06 (0.98–1.16) |
1.10 (0.99–1.23) |
|
Charlson’s Comorbidity Index |
1.00 (0.80–1.24) |
1.11 (0.62–1.97) |
1.29 (0.62–2.67) |
|
BMI |
0.92 (0.82–1.04) |
0.98 (0.75–1.29) |
0.96 (0.69–1.31) |
|
NEWS2 |
1.36 (1.08–1.71)*
|
1.50 (0.79–2.85) |
1.63 (0.80–3.32) |
|
Sex |
|
|
|
|
Men |
1.00 |
1.00 |
1.00 |
|
Women |
0.37 (0.15–0.88)*
|
0.72 (0.09–5.48) |
0.22 (0.01–2.73) |
|
Immunosuppressant use |
|
|
|
|
No |
1.00 |
Not included |
Not included |
|
Yes |
0.68 (0.07–5.94) |
Not included |
Not included |
|
Time from symptom onset to admission |
0.67 (0.48–0.95)*
|
0.37 (0.11–1.28) |
0.50 (0.18–1.37) |
|
COVID-19 vaccination |
|
|
|
|
Inappropriatea
|
1.00 |
1.00 |
1.00 |
|
Appropriateb
|
0.84 (0.33–2.11) |
0.24 (0.02–2.50) |
0.08 (0.00–1.47) |

Acceptability and interruption of nirmatrelvir-ritonavir
We used the medical records to investigate the reasons for not prescribing nirmatrelvir-ritonavir. We could perform this investigation for 75 (19.9%) patients among the 376 patients in the control group. Relative contraindications owing to abnormal liver function or renal function (17/75, 22.7%) were the most common reasons, followed by concomitant use of contraindicated drugs (16/75, 21.3%), patient refusal (15, 20.0%), and mild symptoms (14, 18.7%). Among the 28 patients who discontinued nirmatrelvir-ritonavir medication before completion of the 5-day course, the most common reason for discontinuation was adverse events (20/28, 71.4%), followed by delayed identification of contraindicated concomitant drugs (4/28, 14.3%), patient refusal (3/28, 10.7%), and disease progression (1/28, 3.6%). The adverse events in duplicate included nausea or vomiting (5/20, 25%), a bitter taste (4/20 20%), abdominal pain (3/20, 15%), diarrhea (2/20, 10%), headache (2/20, 10%), high blood pressure (2/20, 10%), and others (one case each of decreased renal function, dizziness, tremor, insomnia, and urticaria).
DISCUSSION
Based on the efficacy of the EPIC-HR trial and the convenient oral formulation, nirmatrelvir-ritonavir was expected to be a game-changer during the COVID-19 pandemic. However, obstacles regarding their major role continue to persist. Real-world evidence is important for policymaking and evidence-based medical practice. In the present study, we explored the effectiveness of nirmatrelvir-ritonavir in preventing the progression to severe disease in high-risk patients with mild-to-moderate COVID-19. Our study showed that nirmatrelvir-ritonavir reduced the need for oxygen therapy by 93% compared to that of the matched control group (aHR, 0.07; 95% CI, 0.01–0.31).
This is the first report on the real-world effectiveness of nirmatrelvir-ritonavir in a population with high vaccine coverage during the omicron variant surge (BA.1 and BA.2 variants) in South Korea. Although the Korean government has been providing nirmatrelvir-ritonavir free of charge for indicated patients with COVID-19, there is only one local study regarding its effectiveness in the literature as of February 2023. Park et al.
16 reported the effectiveness of nirmatrelvir-ritonavir in 819 patients and healthcare workers in five long-term care facilities in Korea between February and April 2022. The study showed that nirmatrelvir-ritonavir reduces severe illness or death by 51% compared to that of the control group (n = 196). However, the patients were not matched, and the definition of the outcome was uncertain.
Other regional studies have confirmed the effectiveness of nirmatrelvir-ritonavir therapy with varying degrees of magnitude. Arbel et al.
17 reported that the nirmatrelvir-ritonavir therapy administered during the omicron variant surge reduced the risk of hospitalization and death in patients with COVID-19 (aged ≥ 65 years) by 73% (aHR, 0.27; 95% CI, 0.15–0.49) and by 79% (aHR, 0.21; 95% CI, 0.05–0.82), respectively, using healthcare data of 109,254 patients with COVID-19 and 3,902 nirmatrelvir-ritonavir-treated patients in Israel. Najjar-Debbiny et al.
18 reported that the nirmatrelvir-ritonavir therapy during the omicron variant surge was associated with a significant decrease in the rate of severe COVID-19 (aHR, 0.54; 95% CI, 0.39–0.75) and that it was more effective in older patients and immunosuppressed patients using also healthcare data of 180,351 patients with COVID-19 and 4,737 nirmatrelvir-ritonavir treated patients in Israel. Wong et al.
19 concluded that nirmatrelvir-ritonavir therapy (n = 890) during the omicron variant surge decreased the need for oxygen therapy in patients with COVID-19 in Hong Kong (HR, 0.73; 95% CI, 0.54–0.97) compared with that of the PS-matched controls. Studies from Israel included only non-hospitalized patients with patient data obtained from a large medical database, and the duration from symptom onset to treatment was not considered in the inclusion criteria. Our study included only hospitalized patients who were not absolutely indicated for in-hospital care due to the national policy on the care for “patients with COVID-19 with comorbidities or vulnerable conditions” in the hospital. Consequently, we suggest that this might have led to a stricter selection of high-risk groups and resulted in a greater protective effect from nirmatrelvir-ritonavir therapy.
Our study also evaluated other relevant factors associated with disease progression and demonstrated that high NEWS2 score at admission, male sex, short duration from symptom onset to admission, and old age were independent risk factors for disease progression. Notably, the female sex was associated with a significantly lower risk (aHR, 0.37) for oxygen support than the male sex. Research on sex disparities regarding COVID-19 infection is limited, but existing data consistently show that the female sex is associated with a lower risk of poor outcomes for COVID-19.
2021 Male sex presented with a high HR of 1.41 (95% CI, 1.13–1.75) in patients aged 40–64 years and 1.65 (95% CI, 1.43–1.91) in patients aged ≥ 65 years for hospitalization due to COVID-19 in a previous study.
17 Given the gender disparities observed, Bienvenu et al.
22 suggested a biological explanation; sexual dimorphism plays a essential role in the genetic and hormonal regulation of immune responses, and it may attribute to the worse outcomes in men with COVID-19. Vaccination status was not a significant protective factor for disease progression in our study. Contrastingly, the prior study
18 performed in Israel revealed that adequate COVID-19 vaccination resulted in a lower risk for severe disease (HR, 0.20; CI, 0.17–0.22) than with the use of nirmatrelvir (HR, 0.54; CI, 0.39–0.75). Our unexpected findings may have resulted from the waning of booster vaccine effectiveness against the omicron variant after 3 months of administration
23 and the small sample size to confirm vaccine effectiveness. In addition, herein, 38.7% of the patients in the inappropriately vaccinated group received two primary vaccine doses, indicating that a significant portion of the group had some level of immunity to COVID-19, which lowered the differences in vaccine effect between the two groups.
We investigated the individual reasons for non-prescription of nirmatrelvir-ritonavir to high-risk patients and discontinuation of nirmatrelvir-ritonavir before completion of the 5-day schedule. Contrary to initial expectations, the overall prescription rate of nirmatrelvir-ritonavir to patients with COVID-19 has been low since its introduction in Korea in January 2022. The government has tried to increase the administration of nirmatrelvir-ritonavir medication in many ways, such as expanding the indicated patient groups, improving the logistics of pharmacies and medical institutions, and educating both patients and physicians in charge of the prescription.
24 However, academic evaluation of the reasons for low prescription has not been adequately performed. Our results showed that severe kidney or liver diseases and the use of contraindicated concomitant medications accounted for > 40% of the non-prescriptions. In addition, patient refusal was the reason for 20% of non-prescriptions. Park et al.
25 reported that 73.2% of 414 patients with COVID-19 who were eligible for nirmatrelvir-ritonavir therapy were not prescribed this medication; the main reason for non-prescription was patient refusal (86.5%), and 15.3% of patients in the treatment group experienced side effects, with 10.8% experiencing gastrointestinal symptoms. Five (4.5%) patients discontinued nirmatrelvir-ritonavir due to the side effects.
This study has several limitations. First, the number of study participants was not sufficient to estimate the effectiveness of the secondary outcome (composite disease progression) or to perform further subgroup analysis. However, our study is still the only matched analysis using real-world data from the South Korean population. Second, the retrospective nature of this cohort study may have affected the quality of the data. Although PS matching was used to balance the risk factors, residual confounding factors remain to be considered. As the study participants were mostly classified as having mild-to-moderate disease, the observational period (hospital length of stay) was short. A policy of home treatment for COVID-19 was implemented during the omicron variant surge, and some patients with uncomplicated infection were discharged before the completion of the obligatory isolation period. Although we could not follow up on the longer outcomes after discharge, the final outcome might not be different, as immediate feedback to the hospital in charge of further medical care was usually handled through government-approved channels. Third, this study enrolled only hospitalized COVID-19 patients. Although hospitalization did not necessarily imply that the patients needed absolute in-hospital care, it categorized them as potentially high-risk patients, requiring hospitalized isolation as per the national policy. Both treatment and control groups were enrolled according to the study inclusion criteria. Therefore, they can be considered equivalent to outpatients. However, this hospitalization process might have led to the selection of patients at a higher risk and may have enhanced the difference in effectiveness. Our study showed a 93% reduction in the need for oxygen therapy with nirmatrelvir-ritonavir usage, in contrast to 89% in the EPIC-HR trial
3 and 46%
18 and 73%
17 in the above-mentioned studies on risk reduction. Lastly, we could collect non-prescription reasons from only 20% out of control group. Although the number of samples were insufficient, our findings are line with the non-prescription reasons from the Government press release or media.
We used the medical records to investigate the reasons for not prescribing nirmatrelvir-ritonavir. We could perform this investigation for 75 (19.9%) patients among the 376 patients in the control group. Relative contraindications owing to abnormal liver function or renal function (17/75, 22.7%) were the most common reasons, followed by concomitant use of contraindicated drugs (16/75, 21.3%), patient refusal (15, 20.0%), and mild symptoms (14, 18.7%).
In conclusion, nirmatrelvir-ritonavir treatment was associated with a 93% reduction in the need for oxygen therapy in high-risk patients with COVID-19 during the omicron variant surge in South Korea. Our study showed higher effectiveness than previous studies on the real-world effectiveness of nirmatrelvir-ritonavir. Patient’s reluctance to consume medication is common, as are the adverse effects of the drug. Physicians are encouraged to actively consider prescribing nirmatrelvir-ritonavir in patients with COVID-19 and to watch out for the adverse effects of the medication. Further follow-up studies reflecting changes in dominant variants, with comprehensive subgroups of patients and alternative antivirals, will help guide national policies and practical medical care.









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download