INTRODUCTION
Coronavirus disease 2019 (COVID-19) continues to spread around the world, and with the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) new variants, the characteristics of the virus are changing.
12 Vaccination programs have been implemented extensively to prevent COVID-19 infection and limit its severity. However, since November 2021, the emergence of the omicron variant has been reported. It is characterized by fast transmission and immune avoidance mechanisms, and cases of reinfection have been reported.
3
The number of confirmed COVID-19 cases has increased rapidly since the omicron BA.1/BA.2 variant become dominant, and related fatality also increased. Among all deaths due to COVID-19 in South Korea, most have occurred during the Omicron BA.1/BA.2 dominance period.
4 Moreover, a survey of cases with COVID-19 has reported high reinfection rates associated with the Omicron variant; the incidence rate has increased from 0.13% during the Delta variant’s dominance period to 0.30% during the omicron BA.1 dominance period and 0.43% during the BA.2 dominance period.
5
Previous studies have reported that the risk of death from reinfection prior to the Omicron epidemic was lower than that of primary infection.
6 However, data are limited on risk factors for death due to reinfection by the omicron variants, delaying the development of policies designed to minimize reinfections and subsequent deaths. Therefore, this study aimed to evaluate characteristics of reinfection with COVID-19 during a period of omicron dominance and assess risk factors for COVID-19-related fatality.
METHODS
Based on information on the current status of confirmed cases reported in the COVID-19 information management system, an investigation of presumed cases of reinfection with COVID-19 was conducted. We compare the epidemiological characteristics between primary infection and presumed cases of reinfection, were based on the weeks in which ≥ 50% of the weekly viral mutation tests were detected according to SARS-CoV-2 mutation analysis report date. Data for the confirmed dates of primary and reinfections, sex, age, residential region, and date of death were extracted from the COVID-19 integrated information system. Data related to COVID-19 vaccination history were retrieved from the national vaccination registration system.
Cases of reinfection were confirmed using polymerase chain reaction, rapid antigen testing, and emergency screening (emergency use approved products) 45 days from the previous date of detection of COVID-19 infection as per the COVID-19 response guidelines. According to the Infectious Disease Prevention and Management Act, death was defined as death after 28 days of follow-up, after excluding foreign personnel, as reported by the COVID-19 Patient Management Information System (Central Disease Control Headquarters).
Statistical analysis
Statistical analyses were performed using the R package (ver.4.0.2; R Foundation for Statistical Computing, Vienna, Austria). In the analysis, the process of excluding primary infection was performed for those who were reinfection. For confirmed cases from January 2, 2022 to July 2, 2022, in order to identify the risk factors for reinfection, it was analyzed with a case-control study design for reinfection.
Statistical analysis is presented as an odds ratio (OR) value using a logistic regression model (
Table 1). To analyze the risk factors of death related to reinfection, forward comparison of case fatality rate was performed. This statistical analysis was also performed using the logistic regression model, but presented as a risk ratio because the study design was cohort (
Table 2). Although it is common to calculate the OR retrospectively, it was intended to calculate the relative risk (RR) of the fatality rate depending on the presence or absence of risk factors. Gender, age, reported area, health status, and vaccination history, which are known risk factors for reinfection, were used for univariate analysis using logistic regression analysis as variables, and multivariate analysis was performed in an enter format after adjusting the variables. Risk factors for reinfection were compared with the reference group within each variable. The risk of reinfection-related death was analyzed by subgroup according to each variable compared to the primary infection. Multivariate logistic regression
7 was used to calculate the OR with 95% confidence intervals (CIs), adjusting for potential confounders (sex, age, reported area, health status, and vaccination history).
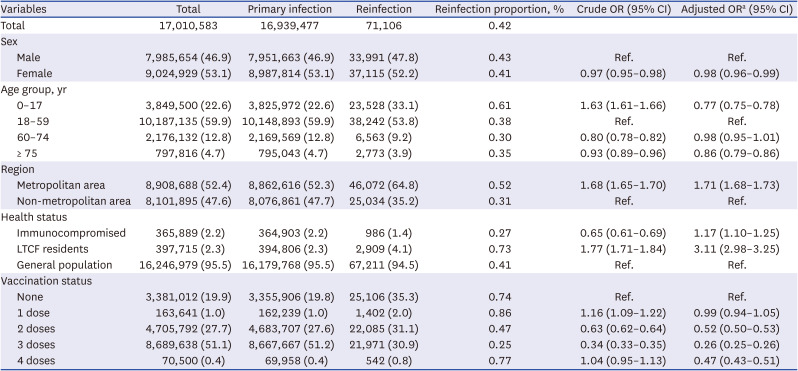
Table 1
Risk factors of severe acute respiratory syndrome coronavirus 2 reinfection cases, January–June 2022, South Korea
|
Variables |
Total |
Primary infection |
Reinfection |
Reinfection proportion, % |
Crude OR (95% CI) |
Adjusted ORa (95% CI) |
|
Total |
17,010,583 |
16,939,477 |
71,106 |
0.42 |
|
|
|
Sex |
|
|
|
|
|
|
|
Male |
7,985,654 (46.9) |
7,951,663 (46.9) |
33,991 (47.8) |
0.43 |
Ref. |
Ref. |
|
Female |
9,024,929 (53.1) |
8,987,814 (53.1) |
37,115 (52.2) |
0.41 |
0.97 (0.95–0.98) |
0.98 (0.96–0.99) |
|
Age group, yr |
|
|
|
|
|
|
|
0–17 |
3,849,500 (22.6) |
3,825,972 (22.6) |
23,528 (33.1) |
0.61 |
1.63 (1.61–1.66) |
0.77 (0.75–0.78) |
|
18–59 |
10,187,135 (59.9) |
10,148,893 (59.9) |
38,242 (53.8) |
0.38 |
Ref. |
Ref. |
|
60–74 |
2,176,132 (12.8) |
2,169,569 (12.8) |
6,563 (9.2) |
0.30 |
0.80 (0.78–0.82) |
0.98 (0.95–1.01) |
|
≥ 75 |
797,816 (4.7) |
795,043 (4.7) |
2,773 (3.9) |
0.35 |
0.93 (0.89–0.96) |
0.86 (0.79–0.86) |
|
Region |
|
|
|
|
|
|
|
Metropolitan area |
8,908,688 (52.4) |
8,862,616 (52.3) |
46,072 (64.8) |
0.52 |
1.68 (1.65–1.70) |
1.71 (1.68–1.73) |
|
Non-metropolitan area |
8,101,895 (47.6) |
8,076,861 (47.7) |
25,034 (35.2) |
0.31 |
Ref. |
Ref. |
|
Health status |
|
|
|
|
|
|
|
Immunocompromised |
365,889 (2.2) |
364,903 (2.2) |
986 (1.4) |
0.27 |
0.65 (0.61–0.69) |
1.17 (1.10–1.25) |
|
LTCF residents |
397,715 (2.3) |
394,806 (2.3) |
2,909 (4.1) |
0.73 |
1.77 (1.71–1.84) |
3.11 (2.98–3.25) |
|
General population |
16,246,979 (95.5) |
16,179,768 (95.5) |
67,211 (94.5) |
0.41 |
Ref. |
Ref. |
|
Vaccination status |
|
|
|
|
|
|
|
None |
3,381,012 (19.9) |
3,355,906 (19.8) |
25,106 (35.3) |
0.74 |
Ref. |
Ref. |
|
1 dose |
163,641 (1.0) |
162,239 (1.0) |
1,402 (2.0) |
0.86 |
1.16 (1.09–1.22) |
0.99 (0.94–1.05) |
|
2 doses |
4,705,792 (27.7) |
4,683,707 (27.6) |
22,085 (31.1) |
0.47 |
0.63 (0.62–0.64) |
0.52 (0.50–0.53) |
|
3 doses |
8,689,638 (51.1) |
8,667,667 (51.2) |
21,971 (30.9) |
0.25 |
0.34 (0.33–0.35) |
0.26 (0.25–0.26) |
|
4 doses |
70,500 (0.4) |
69,958 (0.4) |
542 (0.8) |
0.77 |
1.04 (0.95–1.13) |
0.47 (0.43–0.51) |
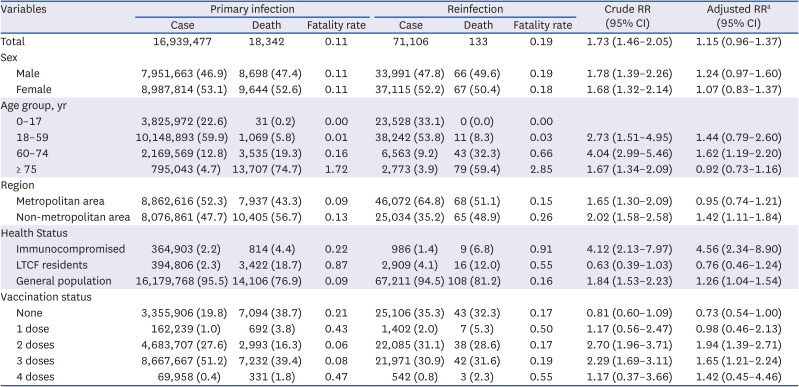
Table 2
Comparison of fatality rates by numbers of coronavirus disease 2019 infections, January–June 2022, South Korea
|
Variables |
Primary infection |
Reinfection |
Crude RR (95% CI) |
Adjusted RRa (95% CI) |
|
Case |
Death |
Fatality rate |
Case |
Death |
Fatality rate |
|
Total |
16,939,477 |
18,342 |
0.11 |
71,106 |
133 |
0.19 |
1.73 (1.46–2.05) |
1.15 (0.96–1.37) |
|
Sex |
|
|
|
|
|
|
|
|
|
Male |
7,951,663 (46.9) |
8,698 (47.4) |
0.11 |
33,991 (47.8) |
66 (49.6) |
0.19 |
1.78 (1.39–2.26) |
1.24 (0.97–1.60) |
|
Female |
8,987,814 (53.1) |
9,644 (52.6) |
0.11 |
37,115 (52.2) |
67 (50.4) |
0.18 |
1.68 (1.32–2.14) |
1.07 (0.83–1.37) |
|
Age group, yr |
|
|
|
|
|
|
|
|
|
0–17 |
3,825,972 (22.6) |
31 (0.2) |
0.00 |
23,528 (33.1) |
0 (0.0) |
0.00 |
|
|
|
18–59 |
10,148,893 (59.9) |
1,069 (5.8) |
0.01 |
38,242 (53.8) |
11 (8.3) |
0.03 |
2.73 (1.51–4.95) |
1.44 (0.79–2.60) |
|
60–74 |
2,169,569 (12.8) |
3,535 (19.3) |
0.16 |
6,563 (9.2) |
43 (32.3) |
0.66 |
4.04 (2.99–5.46) |
1.62 (1.19–2.20) |
|
≥ 75 |
795,043 (4.7) |
13,707 (74.7) |
1.72 |
2,773 (3.9) |
79 (59.4) |
2.85 |
1.67 (1.34–2.09) |
0.92 (0.73–1.16) |
|
Region |
|
|
|
|
|
|
|
|
|
Metropolitan area |
8,862,616 (52.3) |
7,937 (43.3) |
0.09 |
46,072 (64.8) |
68 (51.1) |
0.15 |
1.65 (1.30–2.09) |
0.95 (0.74–1.21) |
|
Non-metropolitan area |
8,076,861 (47.7) |
10,405 (56.7) |
0.13 |
25,034 (35.2) |
65 (48.9) |
0.26 |
2.02 (1.58–2.58) |
1.42 (1.11–1.84) |
|
Health Status |
|
|
|
|
|
|
|
|
|
Immunocompromised |
364,903 (2.2) |
814 (4.4) |
0.22 |
986 (1.4) |
9 (6.8) |
0.91 |
4.12 (2.13–7.97) |
4.56 (2.34–8.90) |
|
LTCF residents |
394,806 (2.3) |
3,422 (18.7) |
0.87 |
2,909 (4.1) |
16 (12.0) |
0.55 |
0.63 (0.39–1.03) |
0.76 (0.46–1.24) |
|
General population |
16,179,768 (95.5) |
14,106 (76.9) |
0.09 |
67,211 (94.5) |
108 (81.2) |
0.16 |
1.84 (1.53–2.23) |
1.26 (1.04–1.54) |
|
Vaccination status |
|
|
|
|
|
|
|
|
|
None |
3,355,906 (19.8) |
7,094 (38.7) |
0.21 |
25,106 (35.3) |
43 (32.3) |
0.17 |
0.81 (0.60–1.09) |
0.73 (0.54–1.00) |
|
1 dose |
162,239 (1.0) |
692 (3.8) |
0.43 |
1,402 (2.0) |
7 (5.3) |
0.50 |
1.17 (0.56–2.47) |
0.98 (0.46–2.13) |
|
2 doses |
4,683,707 (27.6) |
2,993 (16.3) |
0.06 |
22,085 (31.1) |
38 (28.6) |
0.17 |
2.70 (1.96–3.71) |
1.94 (1.39–2.71) |
|
3 doses |
8,667,667 (51.2) |
7,232 (39.4) |
0.08 |
21,971 (30.9) |
42 (31.6) |
0.19 |
2.29 (1.69–3.11) |
1.65 (1.21–2.24) |
|
4 doses |
69,958 (0.4) |
331 (1.8) |
0.47 |
542 (0.8) |
3 (2.3) |
0.55 |
1.17 (0.37–3.66) |
1.42 (0.45–4.46) |
Ethics statement
This study was conducted in accordance with the Infectious Disease Prevention and Control Act (Nos. 12444 and 13392) and approved by the Institutional Bioethics Committee of the Korea Centers for Disease Control and Prevention (2021-12-03-PE-A). Informed consent was submitted by all subjects when they were enrolled.
RESULTS
Presumed cases of reinfection were reported for the first time in 2021, with less than 50 cases per week and less than 0.5% of confirmed cases; however, this number increased with the emergence of the omicron BA.1 variant. The presumed reinfection rate increased rapidly during the BA.2 dominance period and was calculated to be close to 3% (
Fig. 1).
Fig. 1
Weekly distribution of (A) total number of confirmed cases and rate of reinfection; (B) reinfection cases.

Data were available for 17,010,583 confirmed cases of COVID-19 diagnosed between January 2, 2022, and July 2, 2022, the period when the omicron BA.1/BA.2 variant was dominant in South Korea. A total of 4,080 (0.02%) cases were excluded from the analysis because they were admitted to tertiary infections (1,621 cases), the infections were acquired outside the country (2,362 cases), or because of errors in reporting the vaccination history (97 cases). Of the confirmed cases, 99.6% (16,939,477 cases) were primary infections and 0.4% (71,106 cases) were reinfections (
Table 1).
The distribution of men and women in the presumed cases of reinfection was similar. Moreover, age was associated with the proportion of reinfection-presumed cases with 0.61% reinfection in the 0–17 years age group, and 0.38% reinfection in 18–59 years age group. A difference was observed in reinfection based on location with 0.52% reinfection in the metropolitan area, which was higher than the non-metropolitan area (0.31%). Moreover, the proportion of reinfection cases in long-term care facility residents was 0.73%, higher than among immunocompromised persons (0.27%) and the general population (0.41%). When classified according to vaccination history at the time of infection, reinfections were more prevalent among persons with one previous vaccine dose (0.86%) and lowest among those with 3 doses (0.25%) (
Table 1).
Adjusted logistic regression showed women had a slightly lower risk of reinfection than men (adjusted OR [aOR], 0.98; 95% CI, 0.96–0.99). Similarly, the risks of reinfection in the 0–17 years age group (aOR, 0.77; 95% CI, 0.75–0.78) and ≥ 75 years age group (aOR, 0.86; 95% CI, 0.79–0.89) were slightly but significantly lower than in the 18–59 years reference group. Residents of the metropolitan area (aOR, 1.71; 95% CI, 1.68–1.73) had higher reinfection risk than those in the non-metropolitan area. Furthermore, long-term care facility residents (aOR, 3.11; 95% CI, 2.98–3.25) had higher risk than the general population. The risk of reinfection decreased as the number of vaccinations increased; compared to the unvaccinated group, reinfection rates were lower by 48% (aOR, 0.52; 95% CI, 0.80–0.53) in the 2-dose vaccination group, 74% (aOR, 0.26; 95% CI, 0.25–0.26) in the 3-dose vaccination group, and 53% (aOR, 0.47; 95% CI, 0.43–0.51) in the 4-dose vaccination group (
Table 1).
The fatality rate from primary infections was 0.11% and 0.19% from reinfections. The reinfection fatality rates were similar in men and women, and increased with age, which was 2.85% for those ≥ 75 years. The fatality rate in the non-metropolitan area (0.26%) was higher than that in the metropolitan area (0.15%). Moreover, the fatality rate in the immunocompromised group was 0.91%, higher than for long-term care facility residents (0.55%) than in the general population (0.16%). At the time of reinfection, the fatality rate according to vaccination history was 0.55% in the 4-dose vaccination group and 0.50% in the 1-dose vaccination group (
Table 2).
The risk of death was non-significantly higher in the reinfection group than in the primary infection group (crude RR [cRR], 1.73; 95% CI, 1.46–2.05; adjusted RR [aRR], 1.15; 95% CI, 0.96–1.37). The risk of death from reinfection was statistically significantly higher only in the 60–74 years age group (aRR, 1.62; 95% CI, 1.19–2.20). The number of reinfections was the highest in the elderly over 75 years of age group, but the risk of death compared to the primary infection was not statistically significant.
Moreover, in the immunocompromised group (aRR, 4.56; 95% CI, 2.34–8.90), risk of reinfection-related death was statistically significantly higher than that of primary infection, but not in long-term care facility residents. As for the vaccination status, the risk of reinfection-related death was significantly higher in the 2–3 dose vaccination group compared to the primary infection (
Table 2).
DISCUSSION
We identified risk factors for the occurrence of reinfection and death due to reinfection during the dominant period of omicron BA.1/BA.2 variants. As age increased, the proportion of presumed reinfection cases decreased, and the fatality rate increased. In addition, the risk of reinfection decreased as the number of vaccinations increased compared to that in the unvaccinated group.
The omicron variant has higher transmissibility than previous variants and has immune avoidance characteristics, thus, there is a higher risk of reinfection.
8 Similar to previous studies, we found that since 2022, the number of confirmed and reinfected cases in South Korea has increased rapidly compared to the period when the delta variant was dominant, resulting in an increase in the number of deaths.
8910 A systematic review of COVID-19 reinfection characteristics reported that reinfection could occur either due to an insufficient immune response after a primary infection, or due to a mutated virus.
11
According to a study conducted in May–June 2021 in Kentucky, USA, the risk of reinfection is 2.34 times higher in unvaccinated individuals compared to those who are fully vaccinated.
9 In another US study, from March 2020 to December 2021, unvaccinated was associated with an approximately twice as high rate of reinfection compared to fully vaccinated.
12 Since most previous studies have characterized reinfection cases during the delta variant dominance period, it is difficult to directly compare those results with ours. However, similar to previous reports, vaccination had a preventive effect on reinfection, and reinfections increased with this new variant.
In a previous study that analyzed the risk factors for reinfection until April 2022, the risk of occurrence in children and adolescents with limited vaccine coverage was high,
13 but age was not identified as a risk factor for reinfection in this study. The OR values in the 0–17 age group showed different directions before and after adjusted. The interaction between age and other variables was confirmed, but the association was found to be weak. The vaccination coverage for those aged 0–17 was 79.6% for the unvaccinated group (
Supplementary Table 1), which was lower than that of other age groups, so it is thought that the results were reversed after the variable was adjusted. Prior to 2022, the risk of COVID-19 exposure in children and adolescents was low due to strong non-pharmaceutical interventions such as school closures, but the prolonged pandemic may have affected the changed incidence of reinfection.
Studies analyzing vaccination history and fatality has revealed that the fatality rate of vaccinated reinfection is lower than that of unvaccinated-reinfection,
12 and that the risk of death was 70% lower in the 20–59 years age group and 66% lower in those aged ≥ 60 years.
14 In another study that analyzed the risk factors for the severity of reinfection, the risk of death within 28 days of reinfection decreased by 61% compared to that of primary infection,
6 confirming that previous infectivity was associated with a low fatality rate.
In our study, the 4-doses vaccination of reinfection in the elderly over 75 years of age was 5.7%, which was higher than other age groups, and long-term care facility residents was 9.3%, which was higher than other health status groups (
Supplementary Table 1). In a study on the prevention effect of the 4-doses vaccination, the death prevention effect of the 4th vaccination was 62% (34–79%) compared to the 3-doses vaccination for immunocompromised patients and long-term care facility residents in Korea,
15 and 31% (14–45%) of long-term care facility residents in Sweden.
16 The effect of preventing death compared to the 3-doses vaccination of the population in their 60s or older who received the 4-doses vaccination was 76% (48–91%).
17 This can be considered as a low risk of death due to the preventive effect of the 4-doses vaccination.
It can be interpreted as having a high fatality rate in case of reinfection, but in case of reinfection, the detection rate may be reduced, so selective bias may be included. In the case of long-term care facilities with periodic diagnostic tests, it may appear that the reinfection rate is high, but reinfection in the community may be overlooked in many cases. The basis of the quarantine policy is to reduce the severity through reinfection or vaccination.
Research on the rate of reinfection and the fatality rate in case of reinfection shows data in various directions depending on the country of study and research design. As a result of systematic literature review and meta-analysis of the severity of reinfection compared to the initial infection, reinfection did not contribute to the additional risk of death.
18
Our study has several limitations. First, the characteristics of the dominant variant at the time of primary infection or reinfection were not considered. Second, our data did not include information to assess differences by type of vaccine or the period since last vaccination. The generalizability of the results is also limited as vaccination campaigns were not conducted simultaneously for all citizens; however, higher-risk groups were prioritized for vaccination. The proportion of reinfection-presumed cases in the 0–17 years age group is high, but there are no deaths. We think a study is needed to analyze the adult population. Moreover, other factors, including underlying disease, severity of primary infection were not included in the analyses, thus posing residual confounding effect to the result.
As the pandemic continues, the probability of reinfection increases. During the period of omicron dominance, the reinfection rate tended to increase, and the risk of death from reinfection is higher in the immunocompromised group as age increased. In the elderly or immunocompromised, the immune response after vaccination is reduced and the duration is short. In addition, the omicron mutant virus has a high rate of reinfection because it has a good avoidance response. Vaccination can lower the risk of infection, so vaccination for mutated viruses is necessary for the elderly. It is difficult to identify a clear pattern of severity owing to reinfection; therefore, the risk of COVID-19 reinfection needs to be continuously monitored. Vaccination lowers reinfection and severe disease progression, and scheduled vaccinations are important even for those with a history of infection.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download