1. Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980; 303:322–324. PMID:
6991948.

2. Proietti R, Labos C, Davis M, et al. A systematic review and meta-analysis of the association between implantable cardioverter-defibrillator shocks and long-term mortality. Can J Cardiol. 2015; 31:270–277. PMID:
25746019.

3. Mittal S, Movsowitz C, Steinberg JS. Ambulatory external electrocardiographic monitoring: focus on atrial fibrillation. J Am Coll Cardiol. 2011; 58:1741–1749. PMID:
21996384.
4. Mendieta G, Guasch E, Weir D, et al. Advanced interatrial block: a predictor of covert atrial fibrillation in embolic stroke of undetermined source. J Electrocardiol. 2020; 58:113–118. PMID:
31816563.

5. Martínez-Sellés M, Elosua R, Ibarrola M, et al. Advanced interatrial block and P-wave duration are associated with atrial fibrillation and stroke in older adults with heart disease: the BAYES registry. Europace. 2020; 22:1001–1008. PMID:
32449904.
6. Alexander B, Haseeb S, van Rooy H, et al. Reduced P-wave voltage in lead I is associated with development of atrial fibrillation in patients with coronary artery disease. J Atr Fibrillation. 2017; 10:1657. PMID:
29487682.
7. Alexander B, Milden J, Hazim B, et al. New electrocardiographic score for the prediction of atrial fibrillation: the MVP ECG risk score (morphology-voltage-P-wave duration). Ann Noninvasive Electrocardiol. 2019; 24:e12669. PMID:
31184409.

8. McManus DD, Hsu G, Sung SH, et al. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc. 2013; 2:e005694. PMID:
23525446.
9. Sakhuja R, Shah AJ, Keebler M, Thakur RK. Atrial fibrillation in patients with implantable defibrillators. Cardiol Clin. 2009; 27:151–161. PMID:
19111771.
10. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–3726. PMID:
34447992.

11. Kizilirmak F, Demir GG, Gokdeniz T, et al. Changes in electrocardiographic P wave parameters after cryoballoon ablation and their association with atrial fibrillation recurrence. Ann Noninvasive Electrocardiol. 2016; 21:580–587. PMID:
27018476.

12. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021; 42:373–498. PMID:
32860505.
13. Michniewicz E, Mlodawska E, Lopatowska P, Tomaszuk-Kazberuk A, Malyszko J. Patients with atrial fibrillation and coronary artery disease - double trouble. Adv Med Sci. 2018; 63:30–35. PMID:
28818746.

14. Mogensen UM, Jhund PS, Abraham WT, et al. Type of atrial fibrillation and outcomes in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2017; 70:2490–2500. PMID:
29145948.

15. Pistoia F, Sacco S, Tiseo C, Degan D, Ornello R, Carolei A. The Epidemiology of atrial fibrillation and stroke. Cardiol Clin. 2016; 34:255–268. PMID:
27150174.

16. Wang A, Green JB, Halperin JL, Piccini JP Sr. Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol. 2019; 74:1107–1115. PMID:
31439220.
17. Verdecchia P, Angeli F, Reboldi G. Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res. 2018; 122:352–368. PMID:
29348255.
18. Blomström-Lundqvist C, Traykov V, Erba PA, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace. 2020; 22:515–549. PMID:
31702000.

19. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018; 378:417–427. PMID:
29385358.

20. Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008; 51:1357–1365. PMID:
18387436.
21. Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008; 359:1009–1017. PMID:
18768944.

22. Klein RC, Raitt MH, Wilkoff BL, et al. Analysis of implantable cardioverter defibrillator therapy in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. J Cardiovasc Electrophysiol. 2003; 14:940–948. PMID:
12950538.

23. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003; 107:2920–2925. PMID:
12771006.

24. Bunch TJ, Day JD, Olshansky B, Stolen KQ, Mullin CM. INTRINSIC RV Study Investigators. Newly detected atrial fibrillation in patients with an implantable cardioverter-defibrillator is a strong risk marker of increased mortality. Heart Rhythm. 2009; 6:2–8. PMID:
18996055.

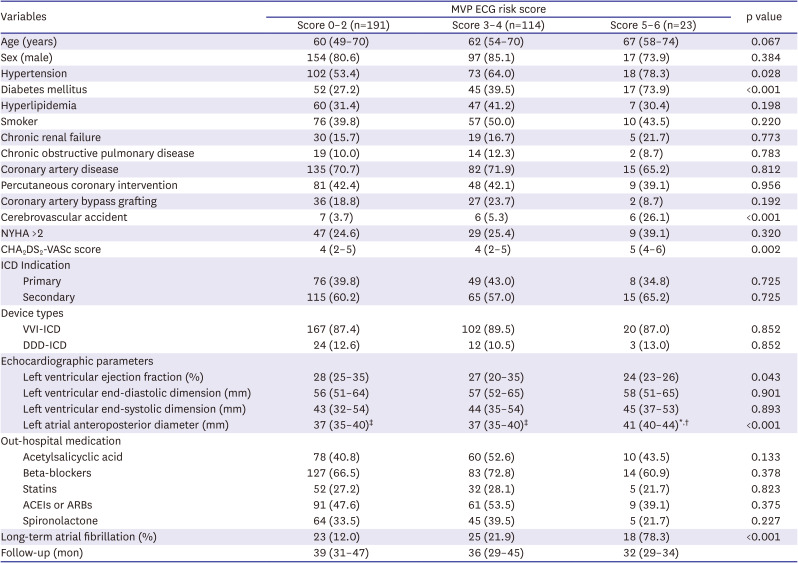
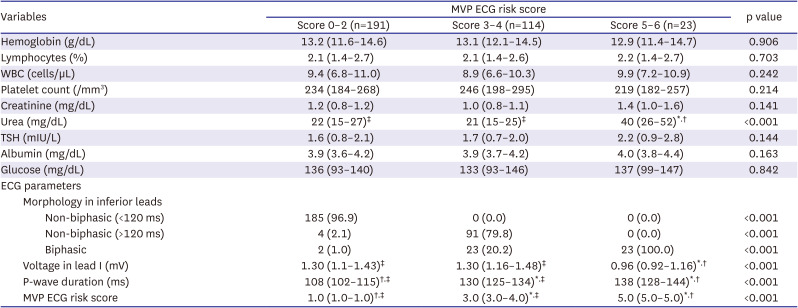
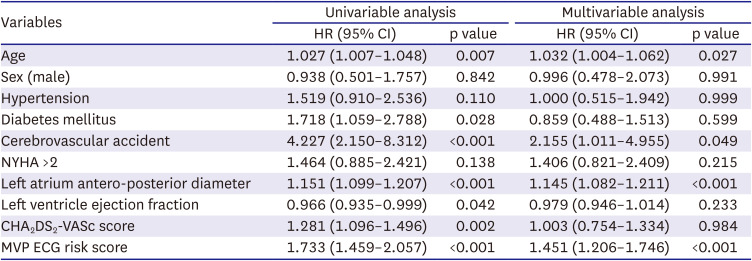
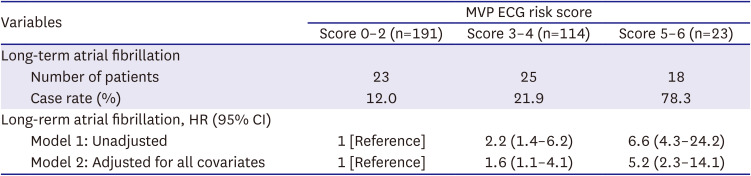





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download