1. Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978; 28:973–977. PMID:
570666.

2. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996; 61:755–759. PMID:
8572814.

3. García-Fernández MA, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol. 2003; 42:1253–1258. PMID:
14522491.

4. Stöllberger C, Schneider B, Finsterer J. Elimination of the left atrial appendage to prevent stroke or embolism? Anatomic, physiologic, and pathophysiologic considerations. Chest. 2003; 124:2356–2362. PMID:
14665520.

5. Yamanaka K, Sekine Y, Nonaka M, et al. Left atrial appendage contributes to left atrial booster function after the maze procedure: quantitative assessment with multidetector computed tomography. Eur J Cardiothorac Surg. 2010; 38:361–365. PMID:
20299235.

6. Yoshihara F, Nishikimi T, Kosakai Y, et al. Atrial natriuretic peptide secretion and body fluid balance after bilateral atrial appendectomy by the maze procedure. J Thorac Cardiovasc Surg. 1998; 116:213–219. PMID:
9699572.

7. Whitlock RP, Belley-Cote EP, Paparella D, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021; 384:2081–2091. PMID:
33999547.

8. Buber J, Luria D, Sternik L, et al. Left atrial contractile function following a successful modified Maze procedure at surgery and the risk for subsequent thromboembolic stroke. J Am Coll Cardiol. 2011; 58:1614–1621. PMID:
21958889.

9. Greenland S, Mickey RM. Re: “The impact of confounder selection criteria on effect estimation”. Am J Epidemiol. 1989; 130:1066.
10. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370–2375. PMID:
11343485.

11. Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004; 110:2287–2292. PMID:
15477396.

12. Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991; 84:527–539. PMID:
1860198.
13. Lee CH, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Left atrial appendage resection versus preservation during the surgical ablation of atrial fibrillation. Ann Thorac Surg. 2014; 97:124–132. PMID:
24075500.

14. Hondo T, Okamoto M, Yamane T, et al. The role of the left atrial appendage. A volume loading study in open-chest dogs. Jpn Heart J. 1995; 36:225–234. PMID:
7596042.

15. Yamamoto N. Experimental study of combined left atrium resection for lung cancer. Nippon Kyobu Geka Gakkai Zasshi. 1986; 34:958–965. PMID:
3772195.
16. Isobe F, Kumano H, Ishikawa T, et al. A new procedure for chronic atrial fibrillation: bilateral appendage-preserving maze procedure. Ann Thorac Surg. 2001; 72:1473–1478. PMID:
11722028.

17. Schneider B, Stollberger C, Sievers HH. Surgical closure of the left atrial appendage - a beneficial procedure? Cardiology. 2005; 104:127–132. PMID:
16118490.

18. Bieging ET, Morris A, Chang L, Dagher L, Marrouche NF, Cates J. Statistical shape analysis of the left atrial appendage predicts stroke in atrial fibrillation. Int J Cardiovasc Imaging. 2021; 37:2521–2527. PMID:
33956285.

19. Ogata J, Yutani C, Otsubo R, et al. Heart and vessel pathology underlying brain infarction in 142 stroke patients. Ann Neurol. 2008; 63:770–781. PMID:
18496843.

20. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020; 141:e139–e596. PMID:
31992061.
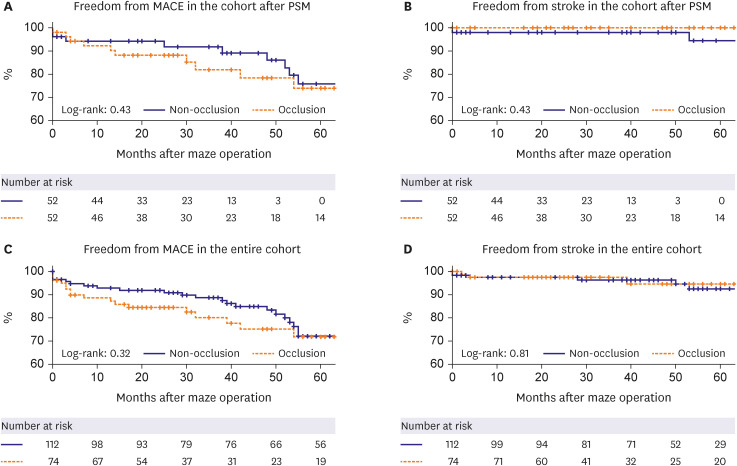
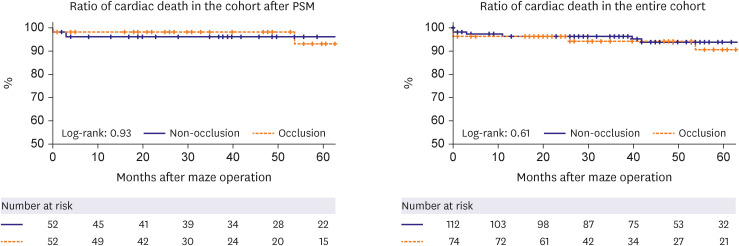
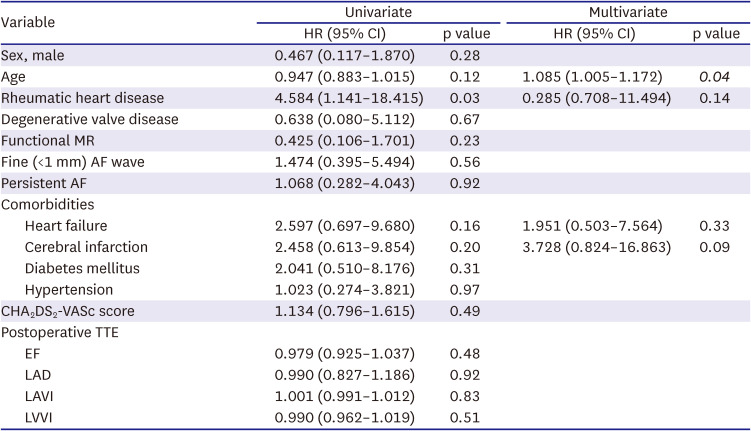




 PDF
PDF Citation
Citation Print
Print



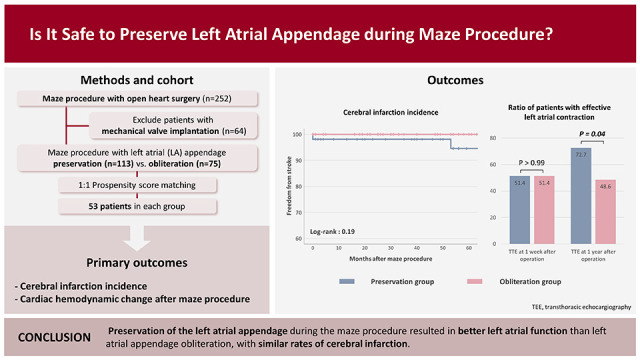
 XML Download
XML Download