DISCUSSION
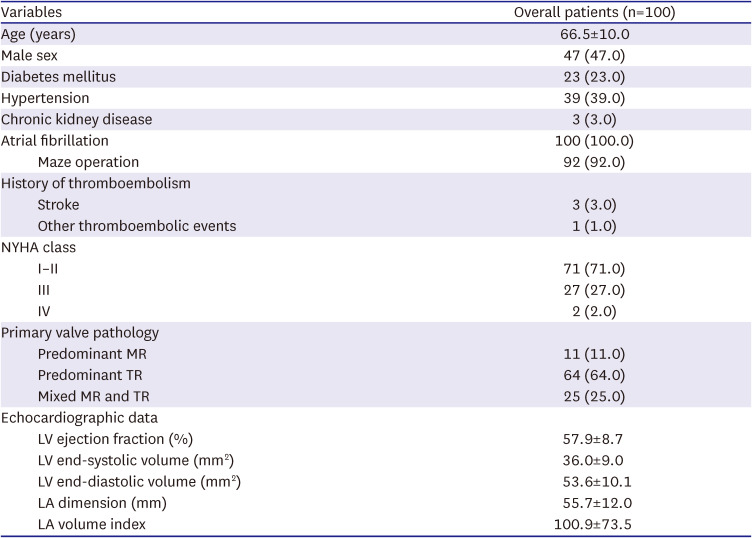
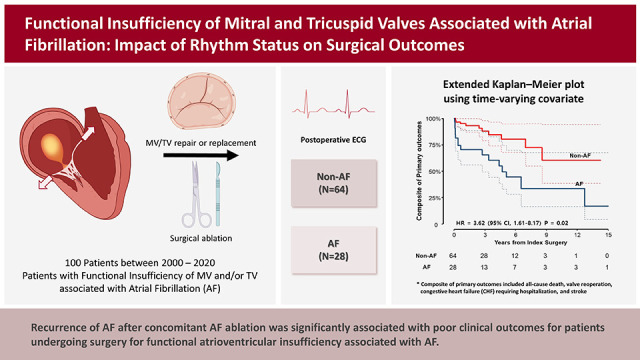
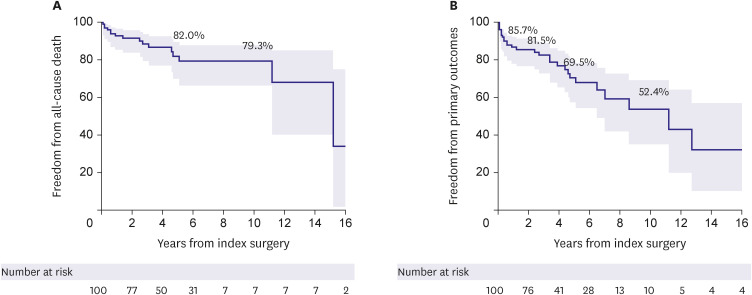
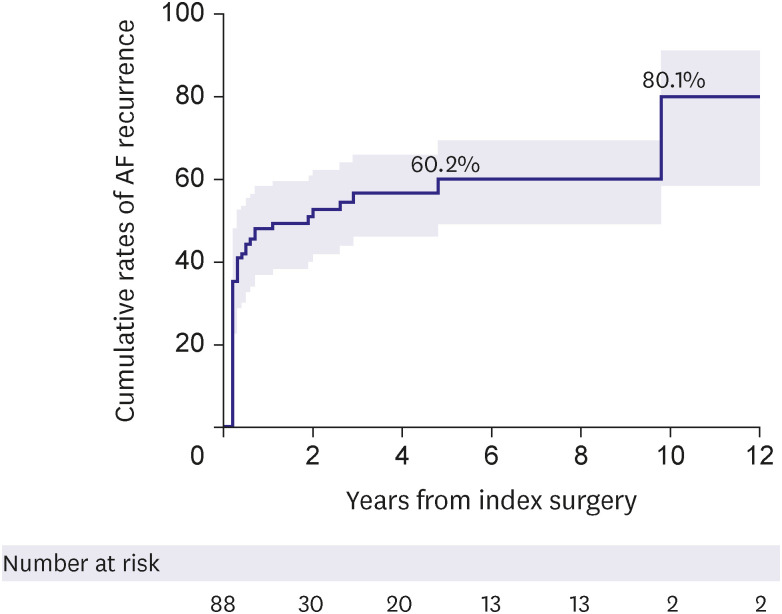
The present study evaluated surgical outcomes of atrial functional atrioventricular insufficiency associated with AF. Despite excellent survival outcomes with no operative mortality and a 10-year survival rate of 79.3% in a cohort with a mean age of 66.5 years, a significant proportion of patients experienced major adverse outcomes such as stroke, CHF, and valve reoperation during long-term follow-up. Moreover, these major adverse events were significantly associated with the recurrence of AF, which was also substantial in its incidence with a cumulative rate of 48.1%, 60.2%, and over 80% at 1 year, 5 years, and beyond 10 years postoperatively. Interestingly, the exclusion of the left atrial auricle (LAA) was significantly protective against the occurrence of primary adverse outcomes, which could be primarily attributed to the lower incidence of stroke compared with preserving LAA.
Functional MRs, in which leaflets of MV are normal, are known to exhibit distinctive pathophysiologic mechanisms in their causes, which have different clinical implications in the corrective intervention of MR.
8) Ventricular functional MR—the most common form of functional MR— is primarily caused by the dilated left ventricle (LV) with consequent systolic tethering of MV leaflets in cases such as ischemic cardiomyopathy and dilated cardiomyopathy.
15) In this disease entity, TEER is suggested as the preferred option over surgery if intervention is deemed necessary due to high estimated surgical risks stemming from poor LV functions.
1) In addition, insufficient evidence for the overt clinical benefits of surgical therapy is another reason for not prioritizing surgery over intervening ventricular functional MR.
16)17)18) In contrast, functional MR may also occur by atrial dilation, termed “atrial functional MR.”
19) Patients with atrial functional MR are mostly associated with longstanding persistent AF with resultant dilatation of the left atrium (LA) and a mitral annulus; these also induce flattening of the anterior mitral leaflet along the mitral annular plane and posterior mitral leaflet bending toward the LV cavity, traditionally referred as the “hamstringing” phenomenon of the posterior mitral leaflet, all of which are conducive to progressive worsening of MR over time despite essentially normal LV leaflets.
5) Given the fairer baseline conditions relative to those with ventricular functional MR and the possibility of eliminating AF—the cause of MR—by the concomitant ablation during surgery, surgery seems to be a more desirable option in atrial functional MR. In particular, as the gap between the anterior and posterior leaflets is more displaced in atrial functional MR by the annular dilatation, capturing these using clipping devices is a technically challenging issue in conducting TEER, which is usually not seen in MR occurring from tethering or prolapse.
19)
Functional TR associated with chronic AF is another disease entity that resembles atrial functional MR. As the fibrous skeleton of the TV annulus is less developed than that of MV, atrial functional insufficiency is more easily provoked in the TV than in the MV by longstanding AF, with much overlap in the pathophysiologic mechanisms between the two.
20) With the same logic as in the atrial functional MR, surgical therapy may be more justifiable than trans-catheter interventions in atrial functional TR, in which interventional therapy for treating TR has also increased.
2)3) With regards to guiding the optimal therapy between surgery and interventional approaches in the atrial functional atrioventricular valve insufficiency, there still are lack of enough data to serve as a reference; therefore, we sought to provide real-world outcomes of surgical therapy, so that the data can be utilized as the benchmark for transcatheter interventions.
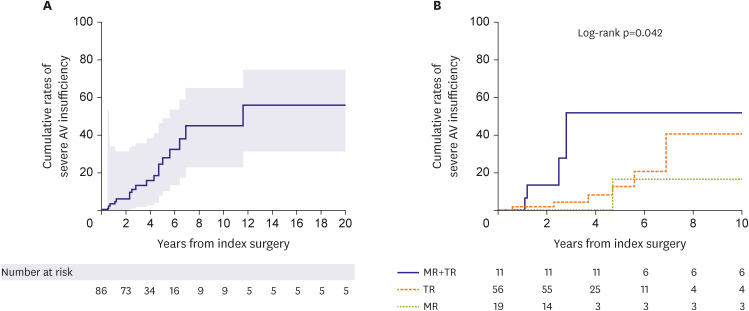
The present study demonstrated excellent results of surgical therapy in terms of survival outcome, demonstrating no operative mortality and a fair long-term survival rate. However, major adverse events represented by a composite primary endpoint were not uncommon, with CHF being the most common form of such events with a 10-year cumulative incidence of 24.5%, followed by stroke (15.1%); during the postoperative 10 years, almost 50% experienced a primary outcome. In addition, echocardiographic assessments in the late postoperative periods showed that 18.9% of patients experienced a severe developing insufficiency in the MV or the TV at 5 years and over 30% at 10 years, indicating that the recurrence of atrioventricular valve insufficiency is quite common and occurs in a time-dependent nature. The seemingly disappointing repair durability relative to those observed from previous studies on degenerative MR (mainly prolapse), including those of our series, may be attributable to the different nature of the pathophysiology between these two disease entities.
21) More importantly, the durability of valve function yielded by surgery should be compared against benchmark TEER, by which a more significant clinical implication can be obtained to guide the optimal therapeutic option.
Given the limited data available for long-term echocardiographic assessments following TEER for atrial functional MR, a recent large-scale study from the Spanish National Registry Transcatheter MV repair showed this issue.
22) In this particular registry, including 1,124 patients undergoing TEER, 48 had atrial functional MR at baseline. At the same time, the degree of atrioventricular valve insufficiency was significantly improved on follow-up echocardiography at 1-year post-TEER, 21.9% still showed advanced MR (grade 3–4), and 33.4% had advanced TR (grade 3–4). Compared with these results from TEER data, the surgical valve durability demonstrated in the present study seems superior, along with excellent early procedural outcomes (i.e., early mortality of 0% in surgery and 2.1% in TEER). Future randomized controlled trials would provide more solid answers for comparative valve durability between surgery and interventional therapy in atrial functional MR.
There has been mounting clinical evidence demonstrating the overt clinical benefit of concomitant AF ablation during cardiac surgery, especially in MV surgery, while the added surgical risks have been perceived to be insignificant.
4) While the well-known clinical benefits of this concomitant AF ablation include improvements in long-term survival and reduction in thromboembolic risks, more favorable MV and TV functions should also be noted.
23)24)25)26)27) The issue of superior atrioventricular valve function by the AF ablation is crucial in atrial functional MR because the AF contributes as the cause of valve failure, and therefore, combining the AF ablation procedure during valve surgery seems an ideal therapeutic approach.
4) The rhythm outcomes, however, seemed relatively poor in the present study. As 92% of patients included in this study underwent concomitant AF ablation, it is regretful that adequate comparisons of outcomes between those with (n=92) and without (n=8) AF ablation are not available due to skewing in the cluster and a small sample size of the “No-ablation” group. Meanwhile, Among those undergoing AF ablation showed high recurrence rates of AF of 48.1% and 60.2% at 1 and 5 years, respectively, which are substantially inferior results as compared with 14.8% and 31.1% in prior studies involving MV for other diseases, such as degenerative prolapse and rheumatic diseases including those from our own experience.
11)25) It could be ascribed to different baseline characteristics, including old age, chronicity of AF, the fine wave pattern of AF, and enlarged atria, which have been identified as important risk factors for the recurrence of post-ablation AF.
28)29) Of note, patients included in the present study were older (67 vs. 54 years, mean) and had a higher proportion of persistent AF (100% vs. 88%) compared to previous studies.
11)25) Also, approximately 20% of patients in this study received left-side-only ablation, which may affect the high incidence of AF recurrence.
Meanwhile, a recent study by Chen et al.
4) is noteworthy for speculating the positive impact of concomitant AF ablation in atrial functional MR. In this cited study, 82 patients with atrial functional MR undergoing MV surgery were included, among whom 52 (63.4%) received concomitant AF ablation.
4) As a result, patients who restored sinus rhythm showed a significantly superior 3-year rate of freedom from recurrent MR (93.8%) compared with those without AF ablation (44.2%; p=0.035).
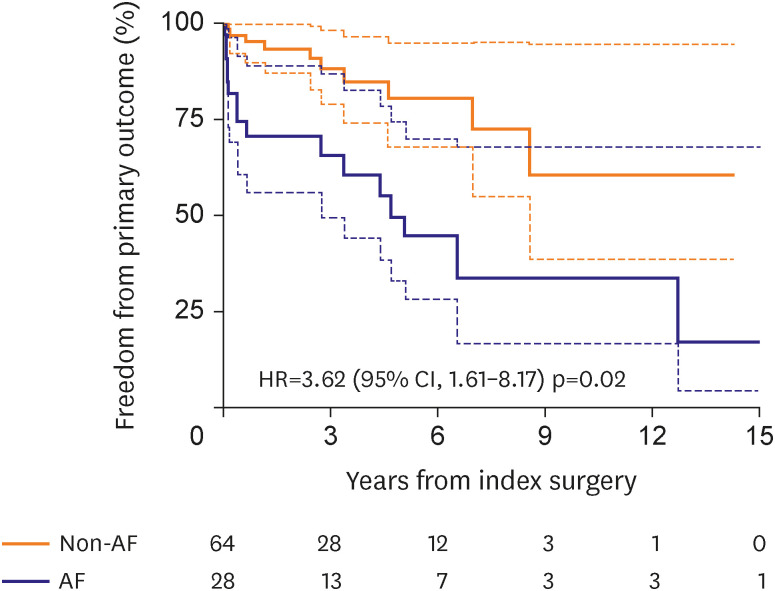
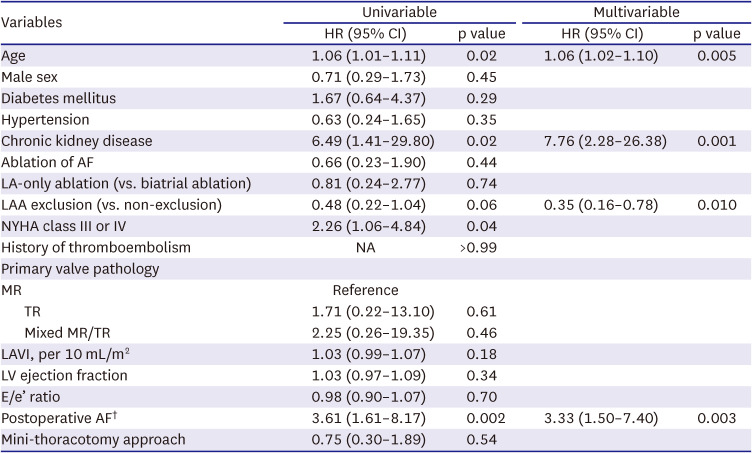
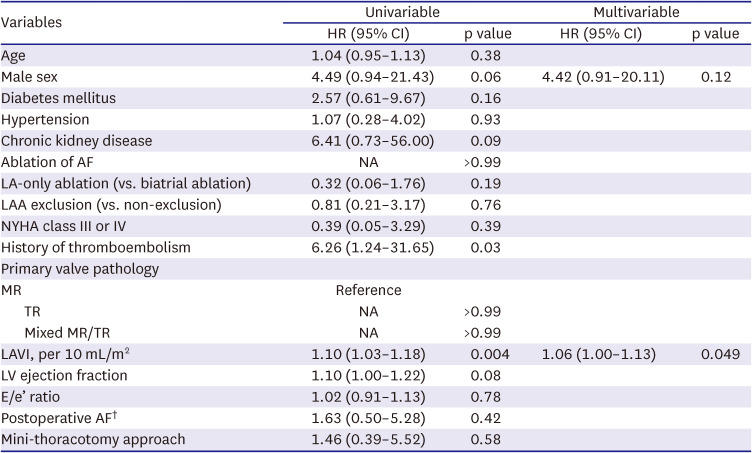
4) However, these results are somewhat discordant from those of our study because the time-varying Cox proportional models in our study did not show a significant impact of postoperative AF on the development of MR/TR but only with a positive trend in their association (HR, 1.63 on univariable model). Unlike the insignificant association between postoperative AF and valve durability, we observed that postoperative AF as a time-varying factor was significantly associated with primary adverse outcomes. This finding is likely suggestive of the beneficial effect of AF ablation despite its relatively low treatment efficacy in eliminating AF in this cohort, which requires further comparative studies on the intention-to-treat basis. In addition, multiple comparative studies on different methods (i.e., limited vs. full lesion sets) of AF ablation in this particular disease entity should be followed, which would be used for guiding the ideal ablation strategy.
In line with the findings of low AF ablation efficacy and the positive association between postoperative AF and adverse outcomes, LAA exclusion seems to be a reasonable strategy in the surgical treatment of atrial functional insufficiency of MV/TV as demonstrated by our study. As the AF ablation efficacy seems suboptimal due to enlarged atria and chronicity of AF, LAA exclusion may be beneficial in preventing thromboembolic complications, and the present study revealed that LAA exclusion was significantly associated with a lower risk of the primary adverse outcome (HR, 0.35; 95% CI, 0.16–0.78; p=0.010) after adjustment for other significant variables in the multivariable model. Among patients undergoing AF ablation (n=92), the primary discriminatory factor for the risk of primary outcomes between those with LAA exclusion (n=58) and preservation (n=32) was a stroke, which occurred in 1 patient in the exclusion group (0.4%/yr) and 4 patients in the preservation group (2.7%/yr). These findings imply that in cases where the risk of AF ablation failure is expected to be as high as in atrial function MR/TR, the LAA exclusion procedure seems a reasonable approach to prevent long-term thromboembolic events. In addition, if a patient is supposed to undergo transcatheter therapy for atrial functional MR/TR associated with AF, utilizing LAA-occluding devices may also be considered based on the findings of this study.
There are several limitations to this study. First, this study was performed retrospectively at a single tertiary center. Possible selection bias in the choice of surgical procedures for valve repair/replacement or surgical ablation may have affected our study results. In addition, the study population is heterogenous, consisting of predominant MR, TR, or mixed; hence, postoperative outcomes and rhythm control status may differ according to the site of predominant functional regurgitation. Furthermore, as this study included patients who received surgical treatment by 7 surgeons over 20 years, unmeasured confounders such as different levels in surgical expertise and learning curves and evolvement of devices may have yet to be fully accounted for. Second, the number of patients included in this study needed to be larger to provide a robust conclusion; therefore, the results should be interpreted as hypothesis-generating.
In conclusion, surgical outcomes of patients with atrial functional atrioventricular insufficiency were favorable; however, a substantial proportion of patients developed AF recurrence over time despite concomitant surgical ablation for AF, which was associated with poor clinical outcomes. Future studies should focus on the development of more effective ablation strategies to further improve the outcomes of these patients.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download