INTRODUCTION
Cases of heart transplantation (HT) bridged with mechanical circulatory support (MCS) have increased globally.
1) Most MCS strategies as a bridge to HT involve the use of durable left ventricular assist devices (LVADs). However, durable LVAD implantation is not generally performed for patients with significant cardiogenic shock, who are typically first treated with temporary MCS.
2)3) Veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) is now one of the most popular temporary MCS approaches and is also used as a bridge to HT.
The use of VA-ECMO as a bridge to HT varies in different countries according to their donor heart utilization and allocation systems. It is known that the incidence of VA-ECMO-bridged HT is 1–5% in the United States,
4) 5–10% in several European countries,
5)6) and around 20% in Italy.
7) In particular, the rate of VA-ECMO bridging is high in Korea, accounting for 25% of all transplanted patients,
8) which may be explained by the highest priority given to ECMO in the allocation system, the strict governmental regulation of access to LVADs, and the relatively short waiting period for transplants.
9)10) However, despite the widespread practice of VA-ECMO-bridged HT, the success of HT and post-transplant survival outcomes among patients bridged with ECMO have been reported to be low.
5)11)12)13)14)
Prolonged VA-ECMO support may jeopardize post-transplant outcomes because of certain intrinsic complications. Peripheral-type VA-ECMO is relatively easy to apply to hemodynamically unstable patients; however, it has disadvantages such as an increased left ventricular (LV) afterload, limb ischemia, bleeding or thrombosis associated with the use of cannulas, and poor mobilization. Because of these non-physiologic effects, peripheral VA-ECMO is regarded as a short-term support option only. Central-type VA-ECMO, in which outflow from the pump directly returns to the ascending aorta, allows antegrade perfusion to end-organs with reduced LV loading, long-standing support with large bore cannulas, and sufficient patient mobilization.
15) However, central-type VA-ECMO also has some disadvantages, such as a high bleeding risk and requirement for sternotomy to insert or remove the cannulas.
16)
Thus far, the data regarding the optimal cannulation strategy for VA-ECMO are limited, especially for patients listed for HT. Most studies comparing the 2 types of VA-ECMO have been confined to cases of post-cardiotomy shock.
15)17) In clinical settings, the VA-ECMO configuration type is determined at the discretion of the clinician with consideration of the type of ECMO that can keep the patient safe until HT. However, the effect of the VA-ECMO configuration on subsequent HT is still largely unknown. Therefore, in this study, a comparison was performed between central and peripheral VA-ECMO to assess their clinical implications in patients when used as a bridge to transplantation.
METHODS
Ethical statement
The study was approved by the Institutional Review Board of Asan medical center (ID: 2022-0311), which waived the requirement for informed consent due to the retrospective nature of the analysis.
Study population and definitions
We examined consecutive HT candidates at a single tertiary center who had been listed as status 0 in the Korean Network for Organ Sharing (KONOS) allocation system between January 2015 and March 2020. The criteria for status 0 in the KONOS allocation system include VA-ECMO, refractory ventricular tachycardia/ventricular fibrillation requiring a temporary ventricular assist device (VAD)/intra-aortic balloon pump (IABP) support, admission to the intensive care unit (ICU) for durable VAD with serious complications (thromboembolism, device infection, mechanical failure, recurrent ventricular arrhythmia), continuous mechanical ventilation due to heart failure, and external VAD implantation (right VAD, LVAD, biventricular assist device).
9) We identified adult heart patients over the age of 18 who were assigned to status 0 on the HT waiting list due to VA-ECMO support. These patients were divided into 2 groups based on the type of VA-ECMO (peripheral group and central group). If there were patients who underwent a change in the configuration, they belonged to the group they were in before HT or death.
The VA-ECMO configuration was defined according to the access for the outflow cannula. Therefore, if outflow was established with a central cannulation technique, it was designated a central type of VA-ECMO even if inflow was achieved using a peripheral cannulation technique. Central cannulation was defined as cannulation involving the aorta for the patient’s arterial outflow and the right atrium or both venae cavae for the patient’s inflow. Peripheral cannulation was defined as cannulation of the femoral and axillary artery for the patient’s arterial outflow and the femoral vein for the patient’s inflow.
9)15) If necessary, left side unloading was performed through left atrial venting via the pulmonary vein or percutaneous septal puncture, or less frequently, through LV apex cannulation.
Bleeding complications during ECMO support were defined as surgical site bleeding that required re-operation in relation to the ECMO process and other events such as psoas muscle spontaneous bleeding or gastrointestinal bleeding. Limb ischemia or vascular access complications were defined as any limb adverse event involving vascular access, including additional use of a distal extremity perfusion cannula, compartment syndrome, dissection, or wound infection. Oxygenator problems referred to all cumbersome complications that necessitated the exchange of the oxygenator (e.g., plasma leakage, clot formation, and membrane dysfunction).
Data collection
This was a retrospective observational study of adult patients receiving VA-ECMO and waiting for HT. Data were collected through a review of their electronic medical records, including demographic characteristics, comorbidities, clinical presentation, medical history, electrocardiographic findings, transthoracic echocardiographic findings, additional treatments, and KONOS status codes for medical urgency at the time when VA-ECMO support had started.
Outcomes
The main outcomes assessed in the analyses were the rate of successful HT and in-hospital all-cause mortality in 3 groups. Additional outcomes included the duration of ECMO support, as well as additional treatments and interventions such as physical rehabilitation, mechanical ventilation support, left side heart unloading, and distal perfusion catheter insertion for lower extremity perfusion. Physical rehabilitation was defined as progressing to bedside cycling or standing. We also evaluated adverse events during ECMO support, such as bleeding, cerebrovascular accident (CVA), bowel ischemia, limb ischemia or vascular access complications, oxygenator problems, and transfusion requirements. Considering that there may be differences in the characteristics of the complications with different ECMO systems, we investigated the corresponding ECMO type and duration when each complication occurred. In order to investigate the prognosis after HT according to the ECMO type, the long-term survival rates of patients who underwent HT were evaluated by subgroup analysis.
Statistical analysis
Continuous variables are presented as means±standard deviations and were analyzed by analysis of variance or Kruskal-Wallis test. Categorical variables are represented as proportions and were compared using the χ2 or Fisher’s exact tests. The significance level was considered as 0.0167 (=0.05/3) for multiple comparisons of the 3 groups by applying the Bonferroni correction method. The time to HT and all-cause mortality at discharge according to ECMO type were estimated with a Cox proportional hazards model. We additionally conducted a landmark analysis for the outcomes of HT and in-hospital mortality. We have made efforts to obtain more accurate and reliable results in comparing the survival outcomes of patients who received central VA ECMO, peripheral VA ECMO, and transition ECMO by reducing the risks of immortal time bias and early death bias through landmark analysis. The landmark time was set to 7 days after the initial ECMO insertion. In order to investigate the prognosis after HT according to ECMO type, the long-term survival rates were conducted as subgroup analysis in the patients who underwent HT. Statistical analyses were performed using the SAS statistical software (version 9.4; SAS Institute, Cary, NC, USA). Statistical analyses were conducted by the Center for Medical Research and Information at Asan Medical Center.
RESULTS
We identified 149 consecutive patients with a status 0 medical urgency who were on the KONOS HT waiting list between January 2015 and March 2020. Among these patients, we excluded 24 pediatric patients under the age of 18, 8 patients who had been weaned off ECMO and then re-evaluated as having a low urgency status due to an improvement in their cardiac function, and 6 patients who had been registered as status 0 for reasons other than VA-ECMO support, such as requiring continuous mechanical ventilation due to heart failure or serious complications of durable VAD requiring ICU admission. Finally, we analyzed a study cohort of 111 adult patients (22.5% female; mean age, 51.3±13.6 years) with status 0 due to a requirement for VA-ECMO support.
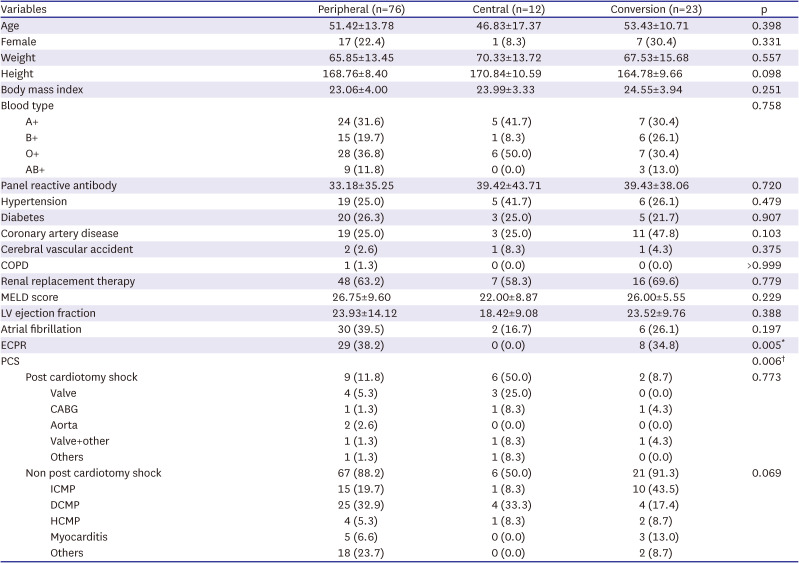
Peripheral VA-ECMO was used in 76 (68.5%) of our study patients, central VA-ECMO was used in 12 (10.8%) cases, and the conversion group contained 23 subjects (20.7%). The baseline characteristics among the 3 groups, other than the underlying etiology who received ECMO support (
Table 1), showed no statistically significant differences. Extracorporeal Cardio-Pulmonary resuscitation was not required among the central group cases, and was not significantly different between the other 2 groups (peripheral 38.2%, central 0.0%, conversion 34.8%, p=0.005). The rate of ECMO support after cardiac surgery was higher in the central group (peripheral 11.8%, central 50.5%, conversion 8.7%, p=0.006).
Table 1
Baseline characteristics of the study patients
|
Variables |
Peripheral (n=76) |
Central (n=12) |
Conversion (n=23) |
p |
|
Age |
51.42±13.78 |
46.83±17.37 |
53.43±10.71 |
0.398 |
|
Female |
17 (22.4) |
1 (8.3) |
7 (30.4) |
0.331 |
|
Weight |
65.85±13.45 |
70.33±13.72 |
67.53±15.68 |
0.557 |
|
Height |
168.76±8.40 |
170.84±10.59 |
164.78±9.66 |
0.098 |
|
Body mass index |
23.06±4.00 |
23.99±3.33 |
24.55±3.94 |
0.251 |
|
Blood type |
|
|
|
0.758 |
|
A+ |
24 (31.6) |
5 (41.7) |
7 (30.4) |
|
B+ |
15 (19.7) |
1 (8.3) |
6 (26.1) |
|
O+ |
28 (36.8) |
6 (50.0) |
7 (30.4) |
|
AB+ |
9 (11.8) |
0 (0.0) |
3 (13.0) |
|
Panel reactive antibody |
33.18±35.25 |
39.42±43.71 |
39.43±38.06 |
0.720 |
|
Hypertension |
19 (25.0) |
5 (41.7) |
6 (26.1) |
0.479 |
|
Diabetes |
20 (26.3) |
3 (25.0) |
5 (21.7) |
0.907 |
|
Coronary artery disease |
19 (25.0) |
3 (25.0) |
11 (47.8) |
0.103 |
|
Cerebral vascular accident |
2 (2.6) |
1 (8.3) |
1 (4.3) |
0.375 |
|
COPD |
1 (1.3) |
0 (0.0) |
0 (0.0) |
>0.999 |
|
Renal replacement therapy |
48 (63.2) |
7 (58.3) |
16 (69.6) |
0.779 |
|
MELD score |
26.75±9.60 |
22.00±8.87 |
26.00±5.55 |
0.229 |
|
LV ejection fraction |
23.93±14.12 |
18.42±9.08 |
23.52±9.76 |
0.388 |
|
Atrial fibrillation |
30 (39.5) |
2 (16.7) |
6 (26.1) |
0.197 |
|
ECPR |
29 (38.2) |
0 (0.0) |
8 (34.8) |
0.005*
|
|
PCS |
|
|
|
0.006†
|
|
Post cardiotomy shock |
9 (11.8) |
6 (50.0) |
2 (8.7) |
0.773 |
|
|
Valve |
4 (5.3) |
3 (25.0) |
0 (0.0) |
|
|
CABG |
1 (1.3) |
1 (8.3) |
1 (4.3) |
|
|
Aorta |
2 (2.6) |
0 (0.0) |
0 (0.0) |
|
|
Valve+other |
1 (1.3) |
1 (8.3) |
1 (4.3) |
|
|
Others |
1 (1.3) |
1 (8.3) |
0 (0.0) |
|
Non post cardiotomy shock |
67 (88.2) |
6 (50.0) |
21 (91.3) |
0.069 |
|
|
ICMP |
15 (19.7) |
1 (8.3) |
10 (43.5) |
|
|
DCMP |
25 (32.9) |
4 (33.3) |
4 (17.4) |
|
|
HCMP |
4 (5.3) |
1 (8.3) |
2 (8.7) |
|
|
Myocarditis |
5 (6.6) |
0 (0.0) |
3 (13.0) |
|
|
Others |
18 (23.7) |
0 (0.0) |
2 (8.7) |
Outcomes
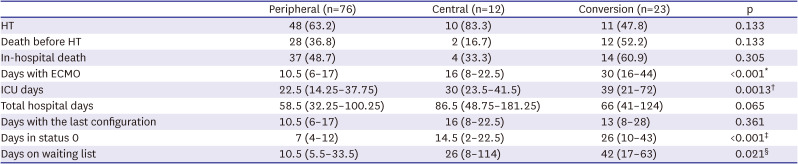
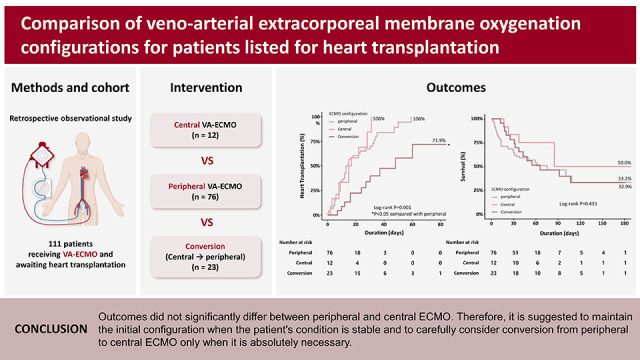
HT was performed in 48 (63.2%) patients in the peripheral group, in 10 (83.3%) patients in the central group, and 11 (47.8%) patients in the conversion group. There was no significant difference between the 3 groups in the number of HT achievement (p=0.133;
Table 2). The median total duration of ECMO was 10.5 (interquartile range [IQR], 6–17), 16 (IQR, 8–22.5), and 30 (IQR, 16–44) days in the peripheral, central and conversion groups, respectively (p<0.001). As a result of this, the cumulative incidence of HT was significantly lower in the conversion group (hazard ratio [HR], 0.292, 95% confidence interval [CI], 0.145–0.586, p=0.001) than in the peripheral (reference) or central groups (
Figure 1A). There was no significant difference found in the cumulative incidence of HT between the peripheral and central groups (HR, 1.206, 95% CI, 0.604–2.409, p=0.595). With regard to all cause in-hospital mortality, there was no difference between the 3 groups (log-rank p=0.433;
Figure 1B). In the results obtained from the landmark analysis, while there was no statistically significant difference in the cumulative incidence of HT among the 3 groups (log-rank p=0.107), the conversion group showed a relatively lower tendency compared to the other 2 groups (
Figure 2A). There was also no difference in in-hospital mortality among the 3 groups in this analysis (log-rank p=0.666;
Figure 2B).
Table 2
Outcomes during ECMO support in status 0 cases
|
Peripheral (n=76) |
Central (n=12) |
Conversion (n=23) |
p |
|
HT |
48 (63.2) |
10 (83.3) |
11 (47.8) |
0.133 |
|
Death before HT |
28 (36.8) |
2 (16.7) |
12 (52.2) |
0.133 |
|
In-hospital death |
37 (48.7) |
4 (33.3) |
14 (60.9) |
0.305 |
|
Days with ECMO |
10.5 (6–17) |
16 (8–22.5) |
30 (16–44) |
<0.001*
|
|
ICU days |
22.5 (14.25–37.75) |
30 (23.5–41.5) |
39 (21–72) |
0.0013†
|
|
Total hospital days |
58.5 (32.25–100.25) |
86.5 (48.75–181.25) |
66 (41–124) |
0.065 |
|
Days with the last configuration |
10.5 (6–17) |
16 (8–22.5) |
13 (8–28) |
0.361 |
|
Days in status 0 |
7 (4–12) |
14.5 (2–22.5) |
26 (10–43) |
<0.001‡
|
|
Days on waiting list |
10.5 (5.5–33.5) |
26 (8–114) |
42 (17–63) |
0.021§
|
Figure 1
Impact of ECMO configuration and conversion on (A) heart transplantation and (B) in-hospital survival outcomes.
ECMO = extracorporeal membrane oxygenation.
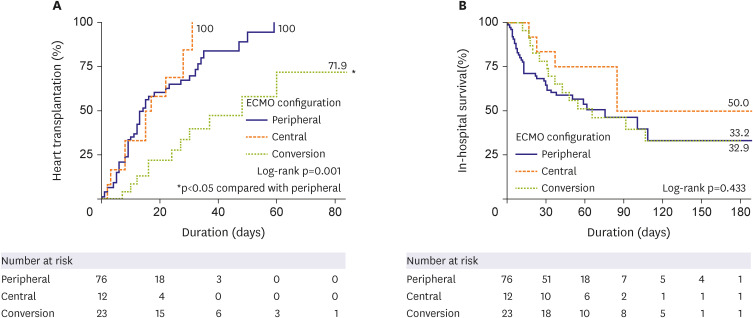
Figure 2
Landmark analysis for impact of ECMO configuration and conversion on (A) heart transplantation and (B) in-hospital survival outcomes.
ECMO = extracorporeal membrane oxygenation.
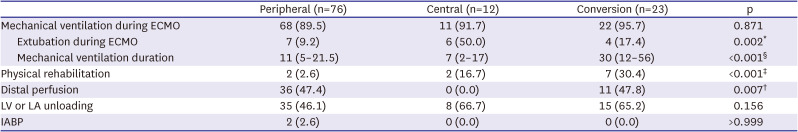
During ECMO support, mechanical ventilation was performed similarly in the 3 groups (peripheral 89.5%, central 91.7%, and conversion 95.7%, p=0.871), but the incidence of extubation (awake ECMO) was significantly higher in the central group (peripheral 9.2%, central 50.0%, conversion 17.4%, p=0.002;
Table 3). In addition, the rate of physical rehabilitation was higher in the conversion group than in the peripheral group (peripheral 2.6%, central 16.7%, conversion 30.4%, p<0.001). Distal perfusion was not done in any case in the central group (peripheral 47.4%, central 8.3%, conversion 47.8%, p<0.001). There were no significant differences in terms of left-side unloading and the use of an IABP among the 3 ECMO groups.
Table 3
Additional treatments and interventions during ECMO support
|
Peripheral (n=76) |
Central (n=12) |
Conversion (n=23) |
p |
|
Mechanical ventilation during ECMO |
68 (89.5) |
11 (91.7) |
22 (95.7) |
0.871 |
|
Extubation during ECMO |
7 (9.2) |
6 (50.0) |
4 (17.4) |
0.002*
|
|
Mechanical ventilation duration |
11 (5–21.5) |
7 (2–17) |
30 (12–56) |
<0.001§
|
|
Physical rehabilitation |
2 (2.6) |
2 (16.7) |
7 (30.4) |
<0.001‡
|
|
Distal perfusion |
36 (47.4) |
0 (0.0) |
11 (47.8) |
0.007†
|
|
LV or LA unloading |
35 (46.1) |
8 (66.7) |
15 (65.2) |
0.156 |
|
IABP |
2 (2.6) |
0 (0.0) |
0 (0.0) |
>0.999 |
Complications
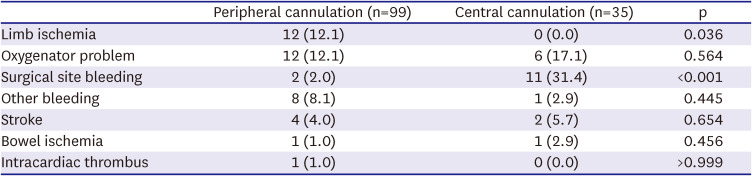
We examined the complications and duration according to the type of ECMO cannulation. Since the ECMO configuration of 23 patients in the central group was converted from peripheral to central, they were added to the peripheral group (n=76); thus, complications were identified for 99 peripheral cases and 35 central cases. Complications did not significantly differ between central and peripheral-type ECMO patients in terms of other bleeding, bowel ischemia, CVA, oxygenator problems, intracardiac thrombus, or transfusion requirements except for limb ischemia and surgical site bleeding. Limb ischemia did not occur in any of the patients in the central group, whereas 12.1% of patients had limb ischemia in the peripheral group (p=0.036). Surgical site bleeding occurred mainly in the central group (31.4%) and only in 3 patients in the peripheral group (2.0%) at the site where coronary artery bypass graft surgery and valve surgery were performed (p<0.001) (
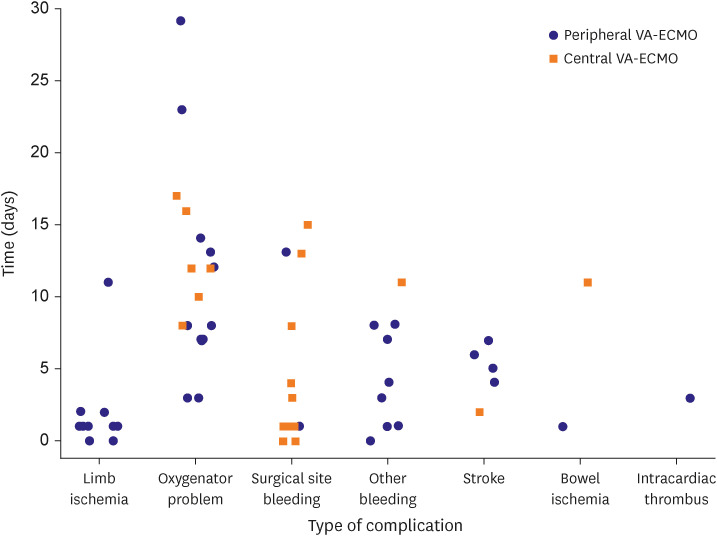
Table 4). When we analyzed the development of complications according to the ECMO type over time, we found that surgical site bleeding and limb ischemia mainly occurred in the early stages after ECMO application, whereas oxygenator problems tended to arise at a later stage (
Figure 3).
Table 4
Complications during extracorporeal membrane oxygenation support
|
Peripheral cannulation (n=99) |
Central cannulation (n=35) |
p |
|
Limb ischemia |
12 (12.1) |
0 (0.0) |
0.036 |
|
Oxygenator problem |
12 (12.1) |
6 (17.1) |
0.564 |
|
Surgical site bleeding |
2 (2.0) |
11 (31.4) |
<0.001 |
|
Other bleeding |
8 (8.1) |
1 (2.9) |
0.445 |
|
Stroke |
4 (4.0) |
2 (5.7) |
0.654 |
|
Bowel ischemia |
1 (1.0) |
1 (2.9) |
0.456 |
|
Intracardiac thrombus |
1 (1.0) |
0 (0.0) |
>0.999 |
Figure 3
Complications during ECMO support.
VA-ECMO = Veno-arterial extracorporeal membrane oxygenation.
Characteristics of the extracorporeal membrane oxygenation conversion group
A total of 23 of our current study patients underwent a change in the ECMO configuration from peripheral to central. Most of these changes had been performed urgently to overcome a worsening clinical status, including pulmonary edema (n=12), lower extremity ischemia or vascular complications (n=4), aortic valve insufficiency (n=1), cardiac tamponade (n=1), and a LV thrombus (n=1). There was only one case in which the conversion to central ECMO was done for active rehabilitation because of the need for prolonged ECMO support prior to simultaneous heart-lung transplantation where finding a matching donor can be difficult. A total of 11 patients in this group finally underwent HT; however, 2 of them died before discharge (
Supplementary Table 1). The median total duration of VA-ECMO support for these patients was 30 days (IQR, 16–44), and the duration of the last configuration was 13 days (IQR, 8–28).
Subgroup analysis
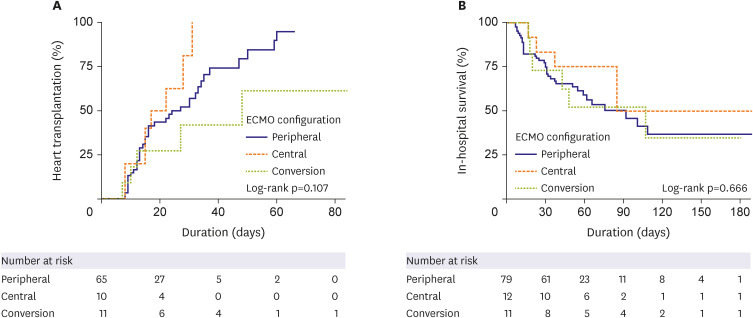
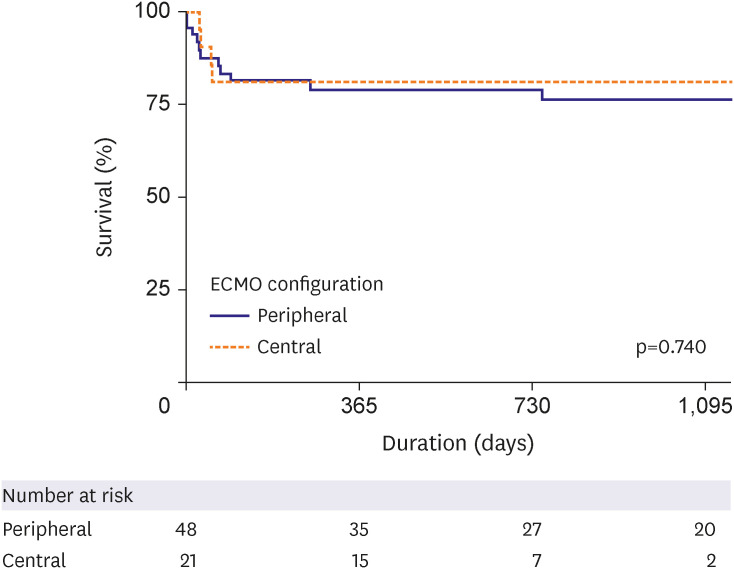
We further analyzed 69 patients who underwent HT for long-term survival as a subgroup analysis. There was also no difference found between the central and peripheral ECMO type in terms of the long-term survival rate in patients who received HT (central vs. peripheral type [reference] HR, 0.825, 95% CI, 0.264–2.579, p=0.740;
Figure 4).
Figure 4
Subgroup analysis of long-term survival in patients who underwent heart transplantation using a Cox proportional hazard model.
ECMO = extracorporeal membrane oxygenation.
DISCUSSION
Our analysis suggests that the likelihood of undergoing HT is higher when patients receive either peripheral or central VA-ECMO support without subsequent changes to this configuration. Notably however, the survival outcomes were found to be comparable between these 2 groups and the cases in our study that had undergone an ECMO conversion. Complication rates were also similar between the 2 ECMO types except for a higher incidence of surgical site bleeding in the central type and a significant occurrence of limb ischemia in the peripheral type cases. To the best of our knowledge, this is the first study to evaluate the prognostic differences between the central and peripheral VA-ECMO configurations of patients waiting for HT.
ECMO-bridged HT was not widely performed in the past and was only used for around 1% of patients awaiting surgery.
1)18) Most patients that have undergone VA-ECMO were classified as INTERMACS 1 or 2, and the accumulated evidence suggests that outcomes are poorer in these cases than in those with a higher grade based on this scoring system.
19) Nevertheless, the use of VA-ECMO prior to HT has been growing, and many countries now give a higher priority for HT to transplant candidates on this bridging treatment than to those on long-term MCS without complications. Since changes to the United Network for Organ Sharing donor allocation system in the United States were made in the fall of 2018, patients on VA-ECMO have had a higher priority for transplantation.
20)21) Some countries, including Korea, have regulated the transition of ECMO to durable LVAD implantation or other temporary MCS interventions such as Impella or CentriMag due to economic burden on the national health insurance system. In addition, there are a considerable number of patients awaiting HT with biventricular failure, who cannot be fully supported by LVADs alone. Therefore, ECMO bridging to transplantation is becoming more popular worldwide.
Nevertheless, VA-ECMO is a bridge therapy; thus, this treatment may be administered in a limited timeframe. Recent registry data indicate that survival rates after VA-ECMO decrease over time, and most recipients have been weaned off or have died by 3 weeks after its introduction.
22) This may be attributed to an increased risk of ECMO complications with cumulative exposure. In our present study, the incidence of complications according to the ECMO type was largely similar, except for limb ischemia and surgical site bleeding. These 2 adverse events were found to arise in the early phase of VA-ECMO, whereas other complications manifested at different times. Hence, greater attention should be paid in the future to the duration of VA-ECMO based on its type.
In comparison with patients receiving peripheral ECMO support, patients receiving central ECMO support required significantly more surgeries to control bleeding complications. They also required more red blood cell and platelet concentrate transfusions; however, these differences were not statistically significant. The results are consistent with the findings of a previous meta-analysis on central ECMO. The reoperation rates of the studies included in the review ranged from 11% to 64%.
15) Among the central ECMO patients in our study, the reoperation rate was 28.6%, indicating a relatively lower risk of bleeding. However, even though the risk of reoperation or transfusion was higher in cases of central ECMO than in cases of peripheral ECMO, there was no significant difference in the in-hospital mortality rate. Therefore, although careful monitoring for reoperation and meticulous hemostasis may be important, the decision to perform central ECMO surgery, if needed, should not be delayed due to fears about bleeding complications.
A total of 23 patients underwent a change in their ECMO configuration. The characteristics of these patients should be reviewed considering the lack of relevant data. By changing from a peripheral to a central ECMO configuration, it has been shown previously that the incidence of pulmonary edema due to elevated LV end-diastolic pressure is reduced,
23) and the extremities become slightly more flexible. These effects would more readily facilitate awake ECMO and bedside rehabilitation for patients awaiting HT. Awake ECMO and the early physical rehabilitation of patients in the ICU are well-known to reduce the frequency of adverse clinical events such as ventilator-associated pneumonia, long-term use of antibiotics, and renal replacement therapy,
21)24) which may improve the safety and longevity of VA-ECMO support. Despite these established benefits of central conversion, we found that the cumulative incidence of HT was significantly lower among patients with a change in the ECMO configuration (conversion group) than among peripheral or central patients without any configuration change (peripheral group, central group) (
Figure 1). Even though we speculate that the poor outcome may be attributed to the urgent conversion of the ECMO type from peripheral to central, there is a limitation in identifying the exact cause of prolonged waiting time. Additionally, it is difficult to determine whether the extended waiting time was solely caused by the deterioration of the patient’s clinical condition or a shortage of suitable donors. Further research and analysis are needed to address this issue.
This study had several limitations. First, this was a retrospective observational study, and the number of study subjects was small. It is possible that there may be some biases in the conversion group, such as protopathic bias, where central conversion attempts to overcome a patient's deteriorating condition may actually increase waiting times for transplantation and lead to unfavorable outcomes, selection bias, where patients with pre-existing poor health may be more likely to undergo conversion due to rapid deterioration, or immortal bias, where patients who survive to conversion may be selectively included. As this is not a randomized trial, there may be limitations in minimizing these biases. However, considering that the conversion group did not show a significant difference in baseline characteristics and that we performed a landmark analysis to adjust for immortal bias, we can cautiously interpret that the result of this study may provide some clinical guidance in determining ECMO configurations for HT-waiting patients. All of factors may have influenced the observed clinical outcomes, and further confirmation of our results in a larger population by randomization is needed. Second, the criteria used to decide on central VA-ECMO were not predetermined, and the decision was made at the discretion of the attending physicians. Third, the lack of information on donor availability during the waiting period and the decision to decline transplantation due to the deterioration of the recipient’s clinical status are limiting factors in fully capturing the multifactorial nature of prolonged waiting times. Fourth, the central VA-ECMO configurations were not analyzed in detail. In most of the central VA-ECMO cases in our current study, a drainage cannula was placed in the left atrial loop or LV apex, and a return cannula was inserted into the ascending aorta. However, we did not perform any comparison according to the cannula position.
In conclusions, we observed no significant difference in the incidence of HT between the peripheral and central groups, although we did note a trend toward lower HT in the conversion group. Therefore, we suggest that if patients are being stably supported with their initial ECMO configuration, whether it is central or peripheral, it should be maintained, and ECMO conversion should only be cautiously performed when necessary.










 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download