1. Mc Namara K, Alzubaidi H, Jackson JK. Cardiovascular disease as a leading cause of death: how are pharmacists getting involved? Integr Pharm Res Pract. 2019; 8:1–11. PMID:
30788283.
2. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019; 139:e56–528. PMID:
30700139.
3. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004; 364:937–952. PMID:
15364185.

4. Cho JH. Sudden death and ventricular arrhythmias in heart failure with preserved ejection fraction. Korean Circ J. 2022; 52:251–264. PMID:
35388994.

5. Tarride JE, Lim M, DesMeules M, et al. A review of the cost of cardiovascular disease. Can J Cardiol. 2009; 25:e195–e202. PMID:
19536390.

6. Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991; 121:293–298. PMID:
1985385.

7. Dahlöf B. Cardiovascular disease risk factors: epidemiology and risk assessment. Am J Cardiol. 2010; 105:3A–9A.

8. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007; 449:804–810. PMID:
17943116.

9. Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006; 312:1355–1359. PMID:
16741115.

10. Heintz-Buschart A, Wilmes P. Human gut microbiome: function matters. Trends Microbiol. 2018; 26:563–574. PMID:
29173869.

11. Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015; 31:69–75. PMID:
25394236.

12. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010; 464:59–65. PMID:
20203603.
13. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010; 90:859–904. PMID:
20664075.

14. Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015; 26:26191. PMID:
25651997.

15. Feng Q, Liu Z, Zhong S, et al. Integrated metabolomics and metagenomics analysis of plasma and urine identified microbial metabolites associated with coronary heart disease. Sci Rep. 2016; 6:22525. PMID:
26932197.

16. Razavi AC, Potts KS, Kelly TN, Bazzano LA. Sex, gut microbiome, and cardiovascular disease risk. Biol Sex Differ. 2019; 10:29. PMID:
31182162.

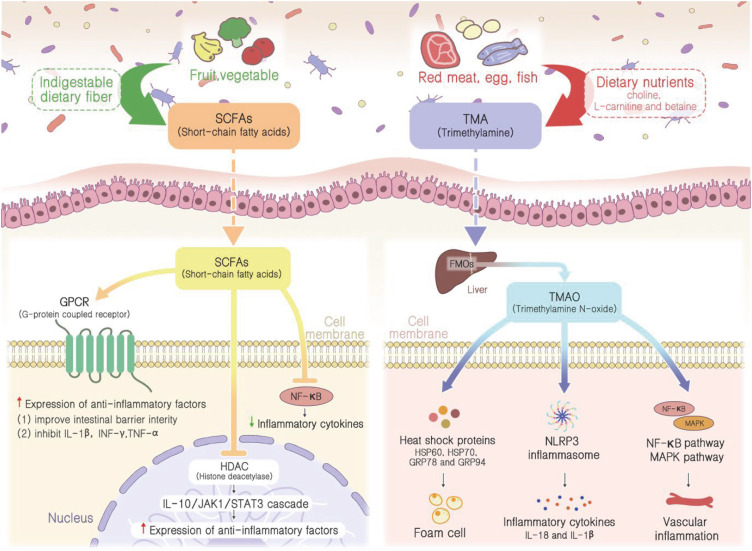
17. Chen XF, Chen X, Tang X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci (Lond). 2020; 134:657–676. PMID:
32219347.

18. Yang S, Li X, Yang F, et al. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol. 2019; 10:1360. PMID:
31803054.

19. Magne F, Gotteland M, Gauthier L, et al. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020; 12:1474. PMID:
32438689.

20. Poll BG, Cheema MU, Pluznick JL. Gut microbial metabolites and blood pressure regulation: focus on SCFAs and TMAO. Physiology (Bethesda). 2020; 35:275–284. PMID:
32490748.

21. Toya T, Corban MT, Marrietta E, et al. Coronary artery disease is associated with an altered gut microbiome composition. PLoS One. 2020; 15:e0227147. PMID:
31995569.

22. Hall AB, Yassour M, Sauk J, et al. A novel
Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017; 9:103. PMID:
29183332.
23. Biddle A, Stewart L, Blanchard J, Leschine S. Untangling the genetic basis of fibrolytic specialization by
Lachnospiraceae and
Ruminococcaceae in diverse gut communities. Diversity (Basel). 2013; 5:627–640.

24. Chen J, Vitetta L. The role of butyrate in attenuating pathobiont-induced hyperinflammation. Immune Netw. 2020; 20:e15. PMID:
32395367.

25. Bach Knudsen KE, Lærke HN, Hedemann MS, et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018; 10:1499. PMID:
30322146.

26. Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016; 23:908–921. PMID:
26947598.

27. Luu M, Pautz S, Kohl V, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. 2019; 10:760. PMID:
30770822.

28. Zhu Q, Gao R, Zhang Y, et al. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol Genomics. 2018; 50:893–903. PMID:
30192713.

29. Zheng YY, Wu TT, Liu ZQ, et al. Gut microbiome-based diagnostic model to predict coronary artery disease. J Agric Food Chem. 2020; 68:3548–3557. PMID:
32100534.

30. Ho KJ, Ramirez JL, Kulkarni R, et al. Plasma gut microbe-derived metabolites associated with peripheral artery disease and major adverse cardiac events. Microorganisms. 2022; 10:2065. PMID:
36296342.

31. Xue H, Chen X, Yu C, et al. Gut microbially produced indole-3-propionic acid inhibits atherosclerosis by promoting reverse cholesterol transport and its deficiency is causally related to atherosclerotic cardiovascular disease. Circ Res. 2022; 131:404–420. PMID:
35893593.

32. Biscetti F, Nardella E, Cecchini AL, Landolfi R, Flex A. The role of the microbiota in the diabetic peripheral artery disease. Mediators Inflamm. 2019; 2019:4128682. PMID:
31205450.

33. Cason CA, Dolan KT, Sharma G, et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg. 2018; 68:1552–1562.e7. PMID:
29248242.

34. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009; 6:306–314. PMID:
19404271.

35. Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013; 18:666–673. PMID:
22688187.

36. Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011; 23:255–264. e119PMID:
21054680.

37. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011; 60:307–317. PMID:
20966022.

38. Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015; 28:203–209. PMID:
25830558.
39. Akhoundzadeh K, Vakili A, Shadnoush M, Sadeghzadeh J. Effects of the oral ingestion of probiotics on brain damage in a transient model of focal cerebral ischemia in mice. Iran J Med Sci. 2018; 43:32–40. PMID:
29398750.
40. Liu J, Sun J, Wang F, et al. Neuroprotective effects of
Clostridium butyricum against vascular dementia in mice via metabolic butyrate. BioMed Res Int. 2015; 2015:412946. PMID:
26523278.
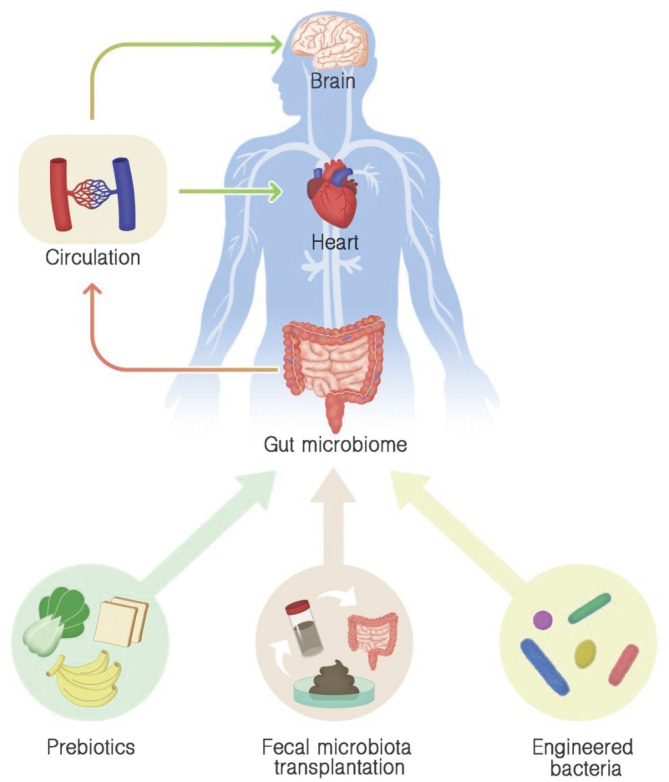
41. Sun J, Wang F, Ling Z, et al.
Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016; 1642:180–188. PMID:
27037183.

42. Rahmati H, Momenabadi S, Vafaei AA, Bandegi AR, Mazaheri Z, Vakili A. Probiotic supplementation attenuates hippocampus injury and spatial learning and memory impairments in a cerebral hypoperfusion mouse model. Mol Biol Rep. 2019; 46:4985–4995. PMID:
31286392.

43. Wanchao S, Chen M, Zhiguo S, Futang X, Mengmeng S. Protective effect and mechanism of
Lactobacillus on cerebral ischemia reperfusion injury in rats. Braz J Med Biol Res. 2018; 51:e7172. PMID:
29791585.

44. Wang Z, Xu K, Zhou H. Characteristics of gut virome and microbiome in patients with stroke. Nan Fang Yi Ke Da Xue Xue Bao. 2021; 41:862–869. PMID:
34238738.
45. Chen L, Shen Y, Wang C, et al.
Megasphaera elsdenii lactate degradation pattern shifts in rumen acidosis models. Front Microbiol. 2019; 10:162. PMID:
30792704.
46. Yin J, Liao SX, He Y, et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015; 4:e002699. PMID:
26597155.

47. O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol. 2016; 7:925. PMID:
27379055.
48. Sokol H, Pigneur B, Watterlot L, et al.
Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008; 105:16731–16736. PMID:
18936492.

49. Lopez-Siles M, Enrich-Capó N, Aldeguer X, et al. Alterations in the abundance and co-occurrence of
Akkermansia muciniphila and
Faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front Cell Infect Microbiol. 2018; 8:281. PMID:
30245977.
50. Tan C, Wu Q, Wang H, et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J Parenter Enteral Nutr. 2021; 45:518–529. PMID:
32473086.

51. Hayashi T, Yamashita T, Watanabe H, et al. Gut microbiome and plasma microbiome-related metabolites in patients with decompensated and compensated heart failure. Circ J. 2018; 83:182–192. PMID:
30487369.

52. Cui X, Ye L, Li J, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018; 8:635. PMID:
29330424.

53. Kamo T, Akazawa H, Suda W, et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS One. 2017; 12:e0174099. PMID:
28328981.

54. Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017; 5:14. PMID:
28143587.

55. Yan Q, Gu Y, Li X, et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017; 7:381. PMID:
28884091.

56. Kim S, Rigatto K, Gazzana MB, et al. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 2020; 75:1063–1071. PMID:
32088998.

57. Zhang Z, Zhang H, Chen T, Shi L, Wang D, Tang D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun Signal. 2022; 20:64. PMID:
35546404.

58. Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021; 13:1–24.

59. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012; 489:242–249. PMID:
22972297.

60. Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics. 2013; 12:489–498. PMID:
23943603.

61. Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017; 52:1–8. PMID:
27448578.

62. Rogler G, Rosano G. The heart and the gut. Eur Heart J. 2014; 35:426–430. PMID:
23864132.

63. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007; 87:1409–1439. PMID:
17928588.

64. Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016; 2016:3094642. PMID:
27110066.

65. Bui TV, Hwang JW, Lee JH, Park HJ, Ban K. Challenges and limitations of strategies to promote therapeutic potential of human mesenchymal stem cells for cell-based cardiac repair. Korean Circ J. 2021; 51:97–113. PMID:
33525065.

66. Usami M, Kishimoto K, Ohata A, et al. Butyrate and trichostatin A attenuate nuclear factor κB activation and tumor necrosis factor α secretion and increase prostaglandin E
2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008; 28:321–328. PMID:
19083427.

67. Li M, van Esch BC, Wagenaar GT, Garssen J, Folkerts G, Henricks PA. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018; 831:52–59. PMID:
29750914.

68. Coutinho-Wolino KS, de F Cardozo LF, de Oliveira Leal V, Mafra D, Stockler-Pinto MB. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur J Nutr. 2021; 60:3567–3584. PMID:
33533968.

69. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011; 472:57–63. PMID:
21475195.

70. Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006; 26:1702–1711. PMID:
16728653.
71. Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013; 368:1575–1584. PMID:
23614584.

72. Lee HY, Lim S, Park S. Role of inflammation in arterial calcification. Korean Circ J. 2021; 51:114–125. PMID:
33525066.

73. Mohammadi A, Gholamhoseyniannajar A, Yaghoobi MM, Jahani Y, Vahabzadeh Z. Expression levels of heat shock protein 60 and glucose-regulated protein 78 in response to trimethylamine-N-oxide treatment in murine macrophage J774A.1 cell line. Cell Mol Biol (Noisy-le-grand). 2015; 61:94–100.
74. Mohammadi A, Vahabzadeh Z, Jamalzadeh S, Khalili T. Trimethylamine-N-oxide, as a risk factor for atherosclerosis, induces stress in J774A.1 murine macrophages. Adv Med Sci. 2018; 63:57–63. PMID:
28822264.

75. Kim HL, Weber T. Pulsatile hemodynamics and coronary artery disease. Korean Circ J. 2021; 51:881–898. PMID:
34595882.

76. Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. IL-1β and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol. 2014; 171:5589–5602. PMID:
25117218.
77. Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. 2016; 5:e002767. PMID:
26903003.

78. Kim HK, Tantry US, Park HW, et al. Ethnic difference of thrombogenicity in patients with cardiovascular disease: a pandora box to explain prognostic differences. Korean Circ J. 2021; 51:202–221. PMID:
33655720.

79. Qiu L, Yang D, Tao X, Yu J, Xiong H, Wei H.
Enterobacter aerogenes ZDY01 attenuates choline-induced trimethylamine
N-oxide levels by remodeling gut microbiota in mice. J Microbiol Biotechnol. 2017; 27:1491–1499. PMID:
28511293.

80. Qiu L, Tao X, Xiong H, Yu J, Wei H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018; 9:4299–4309. PMID:
30039147.

81. Vasu S, Zhou J, Chen J, Johnston PV, Kim DH. Biomaterials-based approaches for cardiac regeneration. Korean Circ J. 2021; 51:943–960. PMID:
34854577.

82. Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol. 2016; 9:229–239.

83. Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory
Clostridium difficile infection. Aliment Pharmacol Ther. 2017; 46:479–493. PMID:
28707337.

84. Hu XF, Zhang WY, Wen Q, et al. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol Res. 2019; 139:412–421. PMID:
30508676.

85. Toral M, Robles-Vera I, de la Visitación N, et al. Critical role of the interaction gut microbiota - sympathetic nervous system in the regulation of blood pressure. Front Physiol. 2019; 10:231. PMID:
30930793.

86. Kim TT, Parajuli N, Sung MM, et al. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am J Physiol Endocrinol Metab. 2018; 315:E511–E519. PMID:
29870676.

87. Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013; 341:1241214. PMID:
24009397.
88. Kim ES, Yoon BH, Lee SM, et al. Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency. Exp Mol Med. 2022; 54:103–114. PMID:
35115674.

89. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012; 143:913–916.e7. PMID:
22728514.

90. Smits LP, Kootte RS, Levin E, et al. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc. 2018; 7:e008342. PMID:
29581220.

91. Fan L, Ren J, Chen Y, et al. Effect of fecal microbiota transplantation on primary hypertension and the underlying mechanism of gut microbiome restoration: protocol of a randomized, blinded, placebo-controlled study. Trials. 2022; 23:178. PMID:
35209934.

92. Zhong HJ, Zeng HL, Cai YL, et al. Washed microbiota transplantation lowers blood pressure in patients with hypertension. Front Cell Infect Microbiol. 2021; 11:679624. PMID:
34458158.

93. Qian B, Zhang K, Li Y, Sun K. Update on gut microbiota in cardiovascular diseases. Front Cell Infect Microbiol. 2022; 12:1059349. PMID:
36439214.

94. Wu H, Chiou J. Potential benefits of probiotics and prebiotics for coronary heart disease and stroke. Nutrients. 2021; 13:2878. PMID:
34445037.

95. Oniszczuk A, Oniszczuk T, Gancarz M, Szymańska J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules. 2021; 26:1172. PMID:
33671813.

96. Kaye DM, Shihata WA, Jama HA, et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. 2020; 141:1393–1403. PMID:
32093510.

97. Rault-Nania MH, Gueux E, Demougeot C, Demigné C, Rock E, Mazur A. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br J Nutr. 2006; 96:840–844. PMID:
17092371.

98. Lim SH. Larch arabinogalactan attenuates myocardial injury by inhibiting apoptotic cascades in a rat model of ischemia-reperfusion. J Med Food. 2017; 20:691–699. PMID:
28622474.

99. Queenan KM, Stewart ML, Smith KN, Thomas W, Fulcher RG, Slavin JL. Concentrated oat beta-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutr J. 2007; 6:6. PMID:
17386092.
100. Tai ES, Fok AC, Chu R, Tan CE. A study to assess the effect of dietary supplementation with soluble fibre (Minolest) on lipid levels in normal subjects with hypercholesterolaemia. Ann Acad Med Singapore. 1999; 28:209–213. PMID:
10497668.
101. Jiang T, Xing X, Zhang L, Liu Z, Zhao J, Liu X. Chitosan oligosaccharides show protective effects in coronary heart disease by improving antioxidant capacity via the increase in intestinal probiotics. Oxid Med Cell Longev. 2019; 2019:7658052. PMID:
30984339.

102. Moludi J, Khedmatgozar H, Nachvak SM, Abdollahzad H, Moradinazar M, Sadeghpour Tabaei A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: a randomized clinical trial. Nutr Neurosci. 2022; 25:1659–1668. PMID:
33641656.

103. Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, et al. A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes. 2017; 125:21–27. PMID:
27219886.

104. Raygan F, Ostadmohammadi V, Asemi Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019; 38:1594–1598. PMID:
30057015.

105. Liu Y, Feng J, Pan H, Zhang X, Zhang Y. Genetically engineered bacterium: principles, practices, and prospects. Front Microbiol. 2022; 13:997587. PMID:
36312915.

106. Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by
Lactococcus lactis secreting interleukin-10. Science. 2000; 289:1352–1355. PMID:
10958782.

107. Yang G, Jiang Y, Yang W, et al. Effective treatment of hypertension by recombinant
Lactobacillus plantarum expressing angiotensin converting enzyme inhibitory peptide. Microb Cell Fact. 2015; 14:202. PMID:
26691527.
108. Müller M, Hernández MA, Goossens GH, et al. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci Rep. 2019; 9:12515. PMID:
31467327.

109. Shubitowski TB, Poll BG, Natarajan N, Pluznick JL. Short-chain fatty acid delivery: assessing exogenous administration of the microbiome metabolite acetate in mice. Physiol Rep. 2019; 7:e14005. PMID:
30810289.

110. Krokowicz L, Stojcev Z, Kaczmarek BF, et al. Microencapsulated sodium butyrate administered to patients with diverticulosis decreases incidence of diverticulitis--a prospective randomized study. Int J Colorectal Dis. 2014; 29:387–393. PMID:
24343275.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download