1. Xiao Y, He C, Chen Y, Ho CT, Wu X, Huang Y, Gao Y, Hou A, Li Z, Wang Y, et al. UPLC-QQQ-MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with
Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022; 378:131999. PMID:
35081481.
2. Xie H, Li X, Ren Z, Qiu W, Chen J, Jiang Q, Chen B, Chen D. Antioxidant and cytoprotective effects of Tibetan tea and its phenolic components. Molecules. 2018; 23:179. PMID:
29364183.

3. Liu Y, Huang W, Zhang C, Li C, Fang Z, Zeng Z, Hu B, Chen H, Wu W, Wang T, et al. Targeted and untargeted metabolomic analyses and biological activity of Tibetan tea. Food Chem. 2022; 384:132517. PMID:
35228002.

4. Wang S, Qiu Y, Gan RY, Zhu F. Chemical constituents and biological properties of Pu-erh tea. Food Res Int. 2022; 154:110899. PMID:
35337597.

5. Zhu J, Yu C, Zhou H, Wei X, Wang Y. Comparative evaluation for phytochemical composition and regulation of blood glucose, hepatic oxidative stress and insulin resistance in mice and HepG2 models of four typical Chinese dark teas. J Sci Food Agric. 2021; 101:6563–6577. PMID:
34018615.

6. Long P, Wen M, Granato D, Zhou J, Wu Y, Hou Y, Zhang L. Untargeted and targeted metabolomics reveal the chemical characteristic of pu-erh tea (
Camellia assamica) during pile-fermentation. Food Chem. 2020; 311:125895. PMID:
31780220.
7. DesRochers N, Walsh JP, Renaud JB, Seifert KA, Yeung KK, Sumarah MW. Metabolomic profiling of fungal pathogens responsible for root rot in American ginseng. Metabolites. 2020; 10:35. PMID:
31947697.

8. Reveglia P, Raimondo ML, Masi M, Cimmino A, Nuzzo G, Corso G, Fontana A, Carlucci A, Evidente A. Untargeted and targeted LC-MS/MS based metabolomics study on
in vitro culture of
Phaeoacremonium species. J Fungi (Basel). 2022; 8:55. PMID:
35049995.

9. Xu J, Liang X, Wang W, Chen S, Tang Q. Study on anti-radiation effect of Ya’an Tibetan tea. J Phys Conf Ser. 2020; 1549:032049.

10. Blaženović I, Kind T, Ji J, Fiehn O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites. 2018; 8:31. PMID:
29748461.

11. Zhou Z, Luo M, Zhang H, Yin Y, Cai Y, Zhu ZJ. Metabolite annotation from knowns to unknowns through knowledge-guided multi-layer metabolic network. bioRxiv. 2022; 6:494523.

12. Zheng Q, Li W, Zhang H, Gao X, Tan S. Optimizing synchronous extraction and antioxidant activity evaluation of polyphenols and polysaccharides from Ya’an Tibetan tea (
Camellia sinensis). Food Sci Nutr. 2019; 8:489–499. PMID:
31993173.

13. Li L, Shi M, Salerno S, Tang M, Guo F, Liu J, Feng Y, Fu M, Huang Q, Ma L, et al. Microbial and metabolomic remodeling by a formula of Sichuan dark tea improves hyperlipidemia in apoE-deficient mice. PLoS One. 2019; 14:e0219010. PMID:
31269076.

14. Xu J, Sun R, Wang J. Human trial of health effects of Tibetan tea. Food Res Dev. 2017; 38:168–171.
15. Tsugawa H, Ikeda K, Takahashi M, Satoh A, Mori Y, Uchino H, Okahashi N, Yamada Y, Tada I, Bonini P, et al. A lipidome atlas in MS-DIAL 4. Nat Biotechnol. 2020; 38:1159–1163. PMID:
32541957.

16. Pandurangan AK, Ismail S, Saadatdoust Z, Esa NM. Allicin alleviates dextran sodium sulfate- (DSS-) induced ulcerative colitis in BALB/c mice. Oxid Med Cell Longev. 2015; 2015:605208. PMID:
26075036.

17. Shi J, Ma W, Wang C, Wu W, Tian J, Zhang Y, Shi Y, Wang J, Peng Q, Lin Z, et al. Impact of various microbial-fermented methods on the chemical profile of dark tea using a single raw tea material. J Agric Food Chem. 2021; 69:4210–4222. PMID:
33792297.

18. Zhu MZ, Li N, Zhou F, Ouyang J, Lu DM, Xu W, Li J, Lin HY, Zhang Z, Xiao JB, et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020; 312:126043. PMID:
31896450.

19. Wang Y, Kan Z, Thompson HJ, Ling T, Ho CT, Li D, Wan X. Impact of six typical processing methods on the chemical composition of tea leaves using a single
Camellia sinensis cultivar, Longjing 43. J Agric Food Chem. 2019; 67:5423–5436. PMID:
30403138.

20. Al-Ishaq RK, Abotaleb M, Kubatka P, Kajo K, Büsselberg D. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019; 9:430. PMID:
31480505.

21. Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, Zhou T. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int J Mol Sci. 2019; 20:4472. PMID:
31510091.

22. Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015; 30:11–26. PMID:
25646037.

23. Nobari H, Saedmocheshi S, Chung LH, Suzuki K, Maynar-Mariño M, Pérez-Gómez J. An overview on how exercise with green tea consumption can prevent the production of reactive oxygen species and improve sports performance. Int J Environ Res Public Health. 2021; 19:218. PMID:
35010479.

24. Mu S, Yang W, Huang G. Antioxidant activities and mechanisms of polysaccharides. Chem Biol Drug Des. 2021; 97:628–632. PMID:
32946177.

25. Song Y, Hui J, Kou W, Xin R, Jia F, Wang N, Hu F, Zhang H, Liu H. Identification of
Inonotus obliquus and analysis of antioxidation and antitumor activities of polysaccharides. Curr Microbiol. 2008; 57:454–462. PMID:
18795365.

26. Hernández Borrero LJ, El-Deiry WS. Tumor suppressor p53: biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer. 2021; 1876:188556. PMID:
33932560.

27. Tu Y, Chen D, Pan T, Chen Z, Xu J, Jin L, Sheng L, Jin X, Wang X, Lan X, et al. Inhibition of miR-431-5p attenuated liver apoptosis through KLF15/p53 signal pathway in S100 induced autoimmune hepatitis mice. Life Sci. 2021; 280:119698. PMID:
34111466.

28. Jazvinšćak Jembrek M, Oršolić N, Mandić L, Sadžak A, Šegota S. Anti-oxidative, anti-inflammatory and anti-apoptotic effects of flavonols: targeting Nrf2, NF-κB and p53 pathways in neurodegeneration. Antioxidants. 2021; 10:1628. PMID:
34679762.

29. Seo DB, Jeong HW, Cho D, Lee BJ, Lee JH, Choi JY, Bae IH, Lee SJ. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J Med Food. 2015; 18:549–556. PMID:
25764354.

30. Wang C, Liu J, Sang S, Ao X, Su M, Hu B, Li H. Effects of tea treatments against high-fat diet-induced disorder by regulating lipid metabolism and the gut microbiota. Comput Math Methods Med. 2022; 2022:9336080. PMID:
35677179.

31. Liu C, Guo Y, Sun L, Lai X, Li Q, Zhang W, Xiang L, Sun S, Cao F. Six types of tea reduce high-fat-diet-induced fat accumulation in mice by increasing lipid metabolism and suppressing inflammation. Food Funct. 2019; 10:2061–2074. PMID:
30907897.

32. Li N, Zhou X, Wang J, Chen J, Lu Y, Sun Y, Song Y, Tan X, Xie G, Chen Y, et al. White tea alleviates non-alcoholic fatty liver disease by regulating energy expenditure and lipid metabolism. Gene. 2022; 833:146553. PMID:
35569768.

33. Hussain K, Yang Y, Wang J, Bian H, Lei X, Chen J, Li Q, Wang L, Zhong Q, Fang X, et al. Comparative study on the weight loss and lipid metabolism by tea polyphenols in diet induced obese C57BL/6J pseudo germ free and conventionalized mice. Food Sci Hum Well. 2022; 11:697–710.

34. Cho D, Jeong HW, Kim JK, Kim AY, Hong YD, Lee JH, Choi JK, Seo DB. Gallocatechin gallate-containing fermented green tea extract ameliorates obesity and hypertriglyceridemia through the modulation of lipid metabolism in adipocytes and myocytes. J Med Food. 2019; 22:779–788. PMID:
31210578.

35. Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011; 22:1–7. PMID:
21115335.

36. Sirichaiwetchakoon K, Lowe GM, Kupittayanant S, Churproong S, Eumkeb G.
Pluchea indica (L.) Less. tea ameliorates hyperglycemia, dyslipidemia, and obesity in high fat diet-fed mice. Evid Based Complement Alternat Med. 2020; 2020:8746137. PMID:
32595747.
37. Wang J, Zheng D, Huang F, Zhao A, Kuang J, Ren Z, Chen T, Lei J, Lin J, Wang X, et al. Theabrownin and
Poria cocos polysaccharide improve lipid metabolism via modulation of bile acid and fatty acid metabolism. Front Pharmacol. 2022; 13:875549. PMID:
35833020.

38. Xu J, Wang W, Liang X, Li P, Zou Y, Du X. Inhibitory effect of the theabrownin and tea polysaccharide extracts of dark tea on lipase. J Phys Conf Ser. 2020; 1549:032048.

39. Giordano Attianese GM, Desvergne B. Integrative and systemic approaches for evaluating PPARβ/δ (PPARD) function. Nucl Recept Signal. 2015; 13:e001. PMID:
25945080.

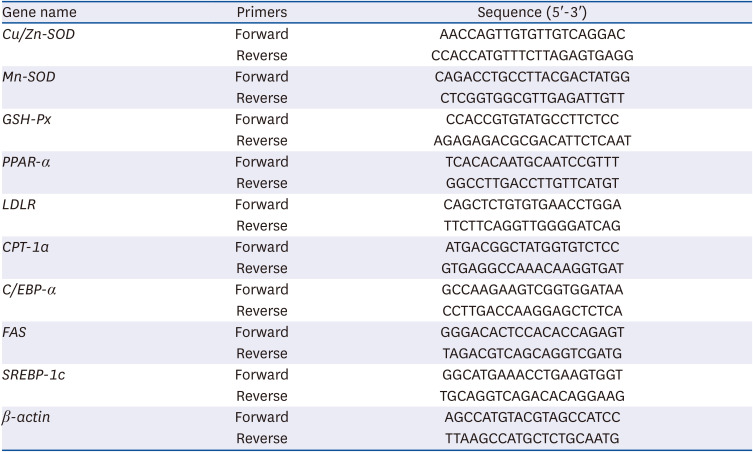
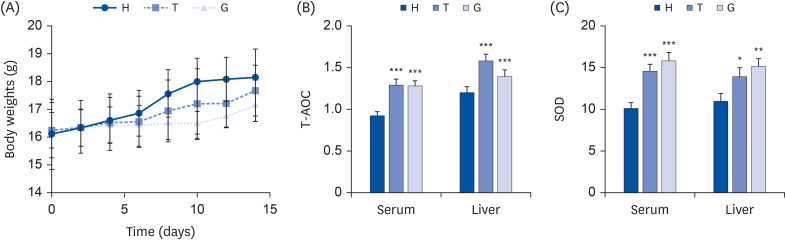
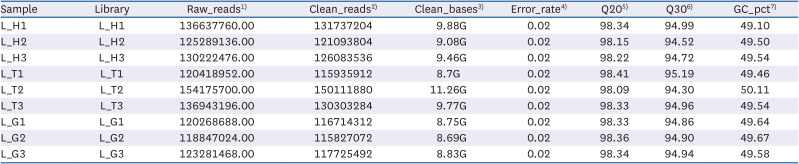
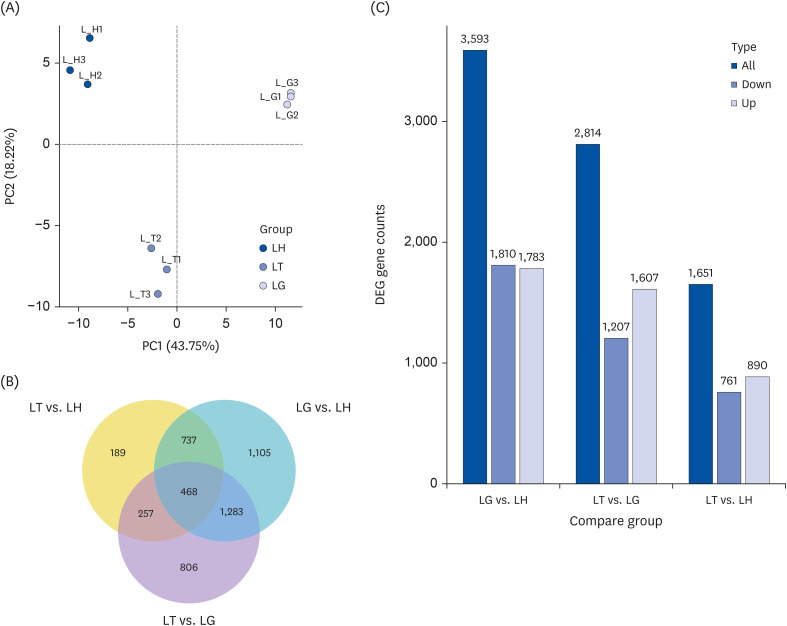




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download