1. Jeszka-Skowron M, Zgoła-Grześkowiak A, Grześkowiak T. Analytical methods applied for the characterization and the determination of bioactive compounds in coffee. Eur Food Res Technol. 2015; 240:19–31.

2. Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017; 359:j5024. PMID:
29167102.

3. Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev. 2017; 26:368–377. PMID:
27111112.

4. Caini S, Cattaruzza MS, Bendinelli B, Tosti G, Masala G, Gnagnarella P, Assedi M, Stanganelli I, Palli D, Gandini S. Coffee, tea and caffeine intake and the risk of non-melanoma skin cancer: a review of the literature and meta-analysis. Eur J Nutr. 2017; 56:1–12.

5. Johnson S, Koh WP, Wang R, Govindarajan S, Yu MC, Yuan JM. Coffee consumption and reduced risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Cancer Causes Control. 2011; 22:503–510. PMID:
21258859.

6. Liu H, Hu GH, Wang XC, Huang TB, Xu L, Lai P, Guo ZF, Xu YF. Coffee consumption and prostate cancer risk: a meta-analysis of cohort studies. Nutr Cancer. 2015; 67:392–400. PMID:
25706900.

7. Wang A, Wang S, Zhu C, Huang H, Wu L, Wan X, Yang X, Zhang H, Miao R, He L, et al. Coffee and cancer risk: a meta-analysis of prospective observational studies. Sci Rep. 2016; 6:33711. PMID:
27665923.

8. Zhou Q, Luo ML, Li H, Li M, Zhou JG. Coffee consumption and risk of endometrial cancer: a dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015; 5:13410. PMID:
26302813.

9. Wijarnpreecha K, Thongprayoon C, Ungprasert P. Coffee consumption and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017; 29:e8–12. PMID:
27824642.

10. Zhang YP, Li WQ, Sun YL, Zhu RT, Wang WJ. Systematic review with meta-analysis: coffee consumption and the risk of gallstone disease. Aliment Pharmacol Ther. 2015; 42:637–648. PMID:
26198295.

11. Liu F, Wang X, Wu G, Chen L, Hu P, Ren H, Hu H. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015; 10:e0142457. PMID:
26556483.

12. Baspinar B, Eskici G, Ozcelik AO. How coffee affects metabolic syndrome and its components. Food Funct. 2017; 8:2089–2101. PMID:
28589997.

13. Grosso G, Micek A, Godos J, Sciacca S, Pajak A, Martínez-González MA, Giovannucci EL, Galvano F. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: a dose-response meta-analysis. Eur J Epidemiol. 2016; 31:1191–1205. PMID:
27699514.

14. Rodríguez-Artalejo F, López-García E. Coffee consumption and cardiovascular disease: a condensed review of epidemiological evidence and mechanisms. J Agric Food Chem. 2018; 66:5257–5263. PMID:
29276945.

15. Wang L, Shen X, Wu Y, Zhang D. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016; 50:228–242. PMID:
26339067.

16. Grosso G, Micek A, Castellano S, Pajak A, Galvano F. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016; 60:223–234. PMID:
26518745.

17. Park KY, Kim HJ, Ahn HS, Kim SH, Park EJ, Yim SY, Jun JB. Effects of coffee consumption on serum uric acid: systematic review and meta-analysis. Semin Arthritis Rheum. 2016; 45:580–586. PMID:
26905267.

18. Peerapen P, Thongboonkerd V. Caffeine in kidney stone disease: risk or benefit? Adv Nutr. 2018; 9:419–424. PMID:
30032225.

19. Alfaro TM, Monteiro RA, Cunha RA, Cordeiro CR. Chronic coffee consumption and respiratory disease: a systematic review. Clin Respir J. 2018; 12:1283–1294. PMID:
28671769.

20. Oñatibia-Astibia A, Martínez-Pinilla E, Franco R. The potential of methylxanthine-based therapies in pediatric respiratory tract diseases. Respir Med. 2016; 112:1–9. PMID:
26880379.

21. Sharif K, Watad A, Bragazzi NL, Adawi M, Amital H, Shoenfeld Y. Coffee and autoimmunity: more than a mere hot beverage! Autoimmun Rev. 2017; 16:712–721. PMID:
28479483.

22. Peng HJ, Chang ZN, Han SH, Won MH, Huang BT. Chemical denaturation of ovalbumin abrogates the induction of oral tolerance of specific IgG antibody and DTH responses in mice. Scand J Immunol. 1995; 42:297–304. PMID:
7544908.

23. Khan DA, Oppenheimer JJ, Lee GB, Fineman SM. Asthma. Ann Allergy Asthma Immunol. 2019; 123:416–417. PMID:
31351982.

24. Kelada SN. Plethysmography phenotype QTL in mice before and after allergen sensitization and challenge. G3 (Bethesda). 2016; 6:2857–2865. PMID:
27449512.

25. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015; 16:45–56. PMID:
25521684.

26. Goto M, Yamaki K, Shinmoto H, Takano-Ishikawa Y. Continuous orally administered coffee enhanced the antigen-specific Th1 response and reduced allergic development in a TCR-transgenic mice model. Biosci Biotechnol Biochem. 2009; 73:2439–2444. PMID:
19897909.

27. Lajunen K, Kalliola S, Kotaniemi-Syrjänen A, Sarna S, Malmberg LP, Pelkonen AS, Mäkelä MJ. Abnormal lung function at preschool age asthma in adolescence? Ann Allergy Asthma Immunol. 2018; 120:520–526. PMID:
29522812.

28. Brigham EP, West NE. Diagnosis of asthma: diagnostic testing. Int Forum Allergy Rhinol. 2015; 5(Suppl 1):S27–S30. PMID:
26335833.

29. Forte GC, da Silva DT, Hennemann ML, Sarmento RA, Almeida JC, de Tarso Roth Dalcin P. Diet effects in the asthma treatment: a systematic review. Crit Rev Food Sci Nutr. 2018; 58:1878–1887. PMID:
28362110.

30. Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther. 2006; 111:877–892. PMID:
16540173.

31. Pohanka M. Caffeine downregulates antibody production in a mouse model. J Appl Biomed. 2014; 13:1–6.

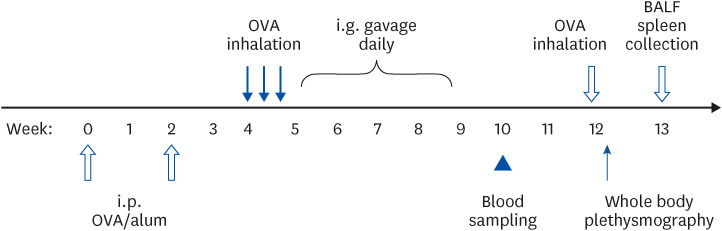
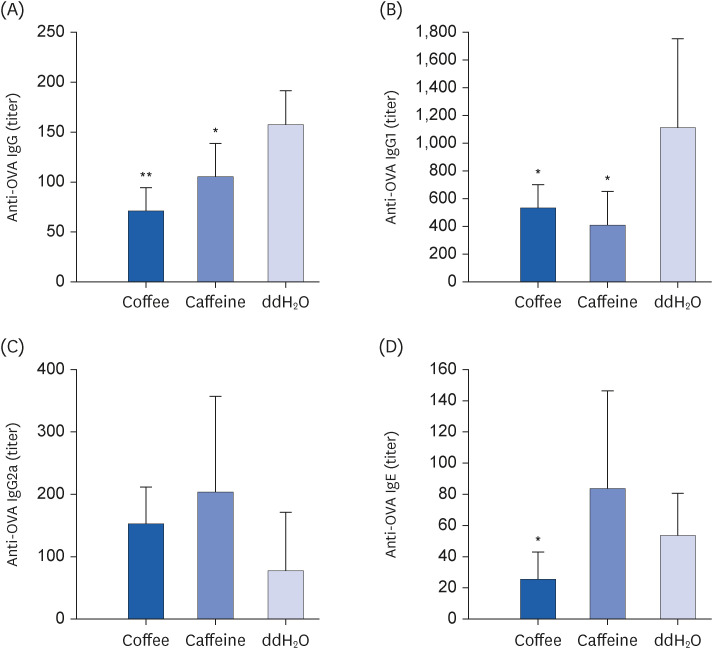
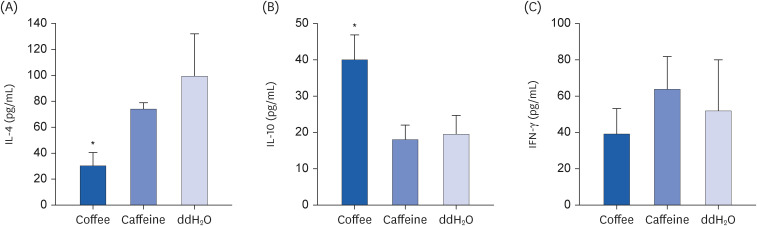
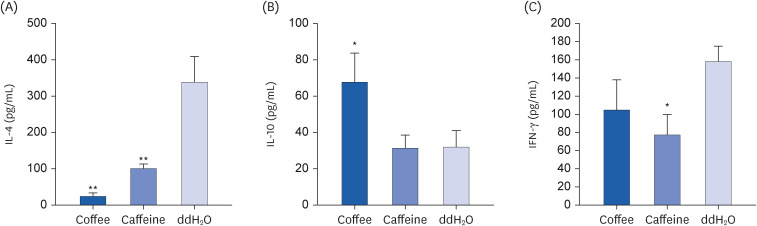




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download