1. Parrado C, Mercado-Saenz S, Perez-Davo A, Gilaberte Y, Gonzalez S, Juarranz A. Environmental stressors on skin aging. Mechanistic insights. Front Pharmacol. 2019; 10:759. PMID:
31354480.

2. Gilchrest BA, Yaar M. Ageing and photoageing of the skin: observations at the cellular and molecular level. Br J Dermatol. 1992; 127(Suppl 41):25–30. PMID:
1390183.

3. Cavinato M, Waltenberger B, Baraldo G, Grade CVC, Stuppner H, Jansen-Dürr P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology. 2017; 18:499–516. PMID:
28702744.

4. Chiang HM, Chen HC, Lin TJ, Shih IC, Wen KC.
Michelia alba extract attenuates UVB-induced expression of matrix metalloproteinases via MAP kinase pathway in human dermal fibroblasts. Food Chem Toxicol. 2012; 50:4260–4269. PMID:
22922035.

5. Hwang E, Park SY, Lee HJ, Lee TY, Sun ZW, Yi TH. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother Res. 2014; 28:1778–1788. PMID:
25131997.

6. Gromkowska-Kępka KJ, Puścion-Jakubik A, Markiewicz-Żukowska R, Socha K. The impact of ultraviolet radiation on skin photoaging - review of
in vitro studies. J Cosmet Dermatol. 2021; 20:3427–3431. PMID:
33655657.
7. Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004; 195:298–308. PMID:
15020192.

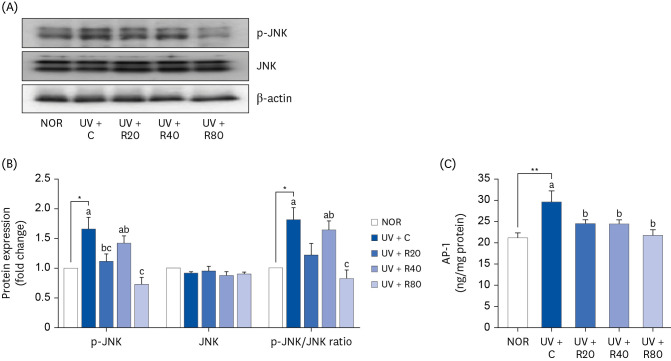
8. Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002; 4:E131–E136. PMID:
11988758.

9. Geoffrey K, Mwangi AN, Maru SM. Sunscreen products: rationale for use, formulation development and regulatory considerations. Saudi Pharm J. 2019; 27:1009–1018. PMID:
31997908.

10. Parrado C, Philips N, Gilaberte Y, Juarranz A, González S. Oral photoprotection: effective agents and potential candidates. Front Med (Lausanne). 2018; 5:188. PMID:
29998107.

11. Cimino F, Cristani M, Saija A, Bonina FP, Virgili F. Protective effects of a red orange extract on UVB-induced damage in human keratinocytes. Biofactors. 2007; 30:129–138. PMID:
18356584.

12. Saija A, Tomaino A, Lo Cascio R, Rapisarda P, Dederen JC.
In vitro antioxidant activity and
in vivo photoprotective effect of a red orange extract. Int J Cosmet Sci. 1998; 20:331–342. PMID:
18505518.

13. Vitali F, Pennisi C, Tomaino A, Bonina F, De Pasquale A, Saija A, Tita B. Effect of a standardized extract of red orange juice on proliferation of human prostate cells
in vitro
. Fitoterapia. 2006; 77:151–155. PMID:
16530345.

14. Bonina FP, Puglia C, Frasca G, Cimino F, Trombetta D, Tringali G, Roccazzello A, Insiriello E, Rapisarda P, Saija A. Protective effects of a standardised red orange extract on air pollution-induced oxidative damage in traffic police officers. Nat Prod Res. 2008; 22:1544–1551. PMID:
19023818.

15. Balestra C, Cimino F, Theunissen S, Snoeck T, Provyn S, Canali R, Bonina A, Virgili F. A red orange extract modulates the vascular response to a recreational dive: a pilot study on the effect of anthocyanins on the physiological consequences of scuba diving. Nat Prod Res. 2016; 30:2101–2106. PMID:
26548425.

16. Tomasello B, Malfa GA, Acquaviva R, La Mantia A, Di Giacomo C. Phytocomplex of a standardized extract from red orange (
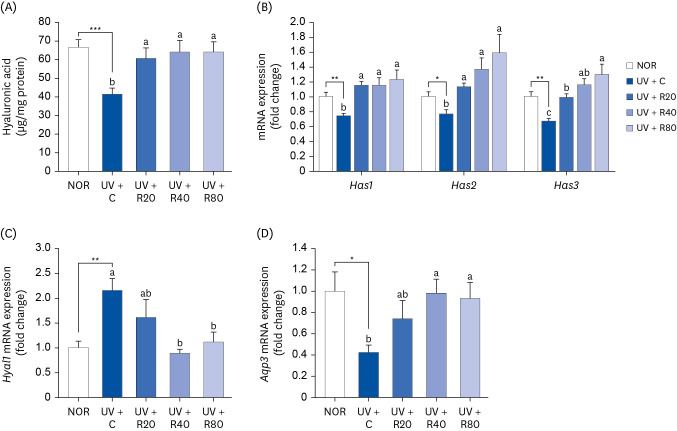
Citrus sinensis L. Osbeck) against photoaging. Cells. 2022; 11:1447. PMID:
35563752.

17. Nobile V, Burioli A, Yu S, Zhifeng S, Cestone E, Insolia V, Zaccaria V, Malfa GA. Photoprotective and antiaging effects of a standardized red orange (
Citrus sinensis (L.) Osbeck) extract in Asian and Caucasian subjects: a randomized, double-blind, controlled study. Nutrients. 2022; 14:2241. PMID:
35684041.

18. Peng LH, Liu S, Xu SY, Chen L, Shan YH, Wei W, Liang WQ, Gao JQ. Inhibitory effects of salidroside and paeonol on tyrosinase activity and melanin synthesis in mouse B16F10 melanoma cells and ultraviolet B-induced pigmentation in guinea pig skin. Phytomedicine. 2013; 20:1082–1087. PMID:
23746955.

19. Lee HS, Lim SM, Jung JI, Kim SM, Lee JK, Kim YH, Cha KM, Oh TK, Moon JM, Kim TY, et al.
Gynostemma pentaphyllum extract ameliorates high-fat diet-induced obesity in C57BL/6N mice by upregulating SIRT1. Nutrients. 2019; 11:2475. PMID:
31618980.

20. Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003; 284:G996–1005. PMID:
12571082.

21. US Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Rockville (MD): US Food and Drug Administration;2005.
22. Yoshizumi M, Nakamura T, Kato M, Ishioka T, Kozawa K, Wakamatsu K, Kimura H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008; 32:1405–1411. PMID:
18782623.

23. Duan X, Wu T, Liu T, Yang H, Ding X, Chen Y, Mu Y. Vicenin-2 ameliorates oxidative damage and photoaging via modulation of MAPKs and MMPs signaling in UVB radiation exposed human skin cells. J Photochem Photobiol B. 2019; 190:76–85. PMID:
30502588.

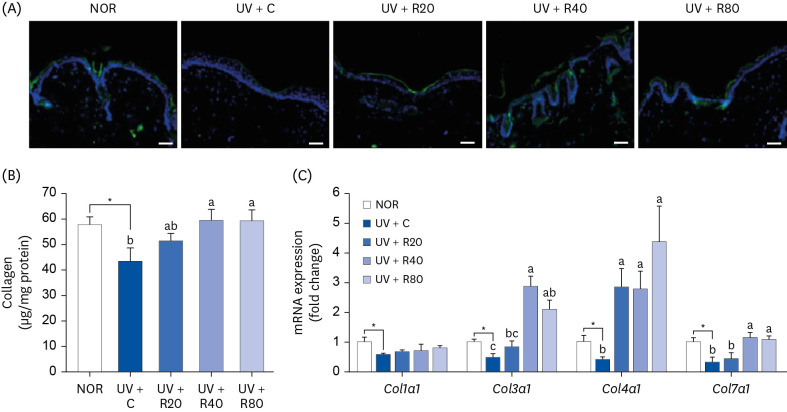
24. Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016; 17:868. PMID:
27271600.

25. Choi SI, Jung TD, Cho BY, Choi SH, Sim WS, Han X, Lee SJ, Kim YC, Lee OH. Anti-photoaging effect of fermented agricultural by-products on ultraviolet B-irradiated hairless mouse skin. Int J Mol Med. 2019; 44:559–568. PMID:
31198982.

26. Young AR. Acute effects of UVR on human eyes and skin. Prog Biophys Mol Biol. 2006; 92:80–85. PMID:
16600340.

27. Saewan N, Jimtaisong A. Natural products as photoprotection. J Cosmet Dermatol. 2015; 14:47–63. PMID:
25582033.

28. Yadav T, Mishra S, Das S, Aggarwal S, Rani V. Anticedants and natural prevention of environmental toxicants induced accelerated aging of skin. Environ Toxicol Pharmacol. 2015; 39:384–391. PMID:
25555260.

29. Grosso G, Galvano F, Mistretta A, Marventano S, Nolfo F, Calabrese G, Buscemi S, Drago F, Veronesi U, Scuderi A. Red orange: experimental models and epidemiological evidence of its benefits on human health. Oxid Med Cell Longev. 2013; 2013:157240. PMID:
23738032.

30. de Lima LP, de Paula BA. A review of the lipolytic effects and the reduction of abdominal fat from bioactive compounds and Moro orange extracts. Heliyon. 2021; 7:e07695. PMID:
34409177.

31. Ribeiro AP, Pereira AG, Todo MC, Fujimori AS, Dos Santos PP, Dantas D, Fernandes AA, Zanati SG, Hassimotto NM, Zornoff LA, et al. Pera orange (
Citrus sinensis) and Moro orange (
Citrus sinensis (L.) Osbeck) juices attenuate left ventricular dysfunction and oxidative stress and improve myocardial energy metabolism in acute doxorubicin-induced cardiotoxicity in rats. Nutrition. 2021; 91-92:111350. PMID:
34265580.
32. Buscemi S, Rosafio G, Arcoleo G, Mattina A, Canino B, Montana M, Verga S, Rini G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am J Clin Nutr. 2012; 95:1089–1095. PMID:
22492368.

33. Magalhães ML, de Sousa RV, Miranda JR, Konig IF, Wouters F, Souza FR, Simão SD, da Silva LA, Nelson DL, das Graças CM. Effects of Moro orange juice (
Citrus sinensis (L.) Osbeck) on some metabolic and morphological parameters in obese and diabetic rats. J Sci Food Agric. 2021; 101:1053–1064. PMID:
32767388.

34. Jiang SJ, Chu AW, Lu ZF, Pan MH, Che DF, Zhou XJ. Ultraviolet B-induced alterations of the skin barrier and epidermal calcium gradient. Exp Dermatol. 2007; 16:985–992. PMID:
18031457.

35. Haratake A, Uchida Y, Schmuth M, Tanno O, Yasuda R, Epstein JH, Elias PM, Holleran WM. UVB-induced alterations in permeability barrier function: roles for epidermal hyperproliferation and thymocyte-mediated response. J Invest Dermatol. 1997; 108:769–775. PMID:
9129231.

36. Mojumdar EH, Pham QD, Topgaard D, Sparr E. Skin hydration: interplay between molecular dynamics, structure and water uptake in the stratum corneum. Sci Rep. 2017; 7:15712. PMID:
29146971.

37. Dai G, Freudenberger T, Zipper P, Melchior A, Grether-Beck S, Rabausch B, de Groot J, Twarock S, Hanenberg H, Homey B, et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am J Pathol. 2007; 171:1451–1461. PMID:
17982124.

38. Yun MS, Kim C, Hwang JK.
Agastache rugosa Kuntze attenuates UVB-induced photoaging in hairless mice through the regulation of MAPK/AP-1 and TGF-β/Smad pathways. J Microbiol Biotechnol. 2019; 29:1349–1360. PMID:
31474086.

39. Razia S, Park H, Shin E, Shim KS, Cho E, Kim SY. Effects of
Aloe vera flower extract and its active constituent isoorientin on skin moisturization via regulating involucrin expression:
in vitro and molecular docking studies. Molecules. 2021; 26:2626. PMID:
33946287.

40. Lee HJ, Im AR, Kim SM, Kang HS, Lee JD, Chae S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. BMC Complement Altern Med. 2018; 18:39. PMID:
29382339.

41. Park JE, Pyun HB, Woo SW, Jeong JH, Hwang JK. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol Photoimmunol Photomed. 2014; 30:237–245. PMID:
24313661.

42. Han HS, Shin JS, Myung DB, Ahn HS, Lee SH, Kim HJ, Lee KT.
Hydrangea serrata (Thunb.) Ser. extract attenuate UVB-induced photoaging through MAPK/AP-1 inactivation in human skin fibroblasts and hairless mice. Nutrients. 2019; 11:553. PMID:
30841605.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download