Abstract
Soft tissue defects can occur in the head and neck due to various causes, and head and neck surgery is often performed to reconstruct soft tissue defects. However, head and neck reconstruction remains delicate and complex as a surgical procedure. The reconstruction of a large defect at the base of the skull, especially after resection of cancer in the anterior base of the skull that has invaded adjacent tissues, is particularly difficult. We present a case of successful reconstruction of a large skull base defect using an anterolateral thigh (ALT) free flap and galeal flap division after tumor resection in the anterior skull base. Paranasal sinus cancer involving the bilateral frontoethmoidal sinuses was resected, and an anterior skull base defect was noted, with communication between the intracranium and nasal cavity and a skin defect at the glabella. A galeal flap was divided to create an anatomical and functional barrier to communication between the nasal cavity and intracranium. The soft tissue defect at the anterior skull base was then reconstructed using an ALT free flap containing the vastus lateralis muscle, and the skin defect at the glabella was covered. No postoperative complications, such as cerebrospinal fluid leakage, developed. The reconstructed flap remained intact after subsequent radiation therapy. Based on this study, we propose that using a galeal flap and ALT free flap in a large skull base defect can yield a robust flap that can endure postoperative radiotherapy with a minimal risk of complications.
Soft tissue defects can occur in the head and neck, particularly at the anterior base of the skull, for various reasons, including tumors, trauma, and infection. Sinonasal cancers are rare, accounting for 3% to 5% of head and neck cancers and less than 1% of all cancers. Most of these tumors are difficult to diagnose because they are often asymptomatic and discovered at a locally advanced stage. Surgery plays an important role in the treatment of these tumors and is considered the gold standard treatment when feasible, possibly complemented by adjuvant therapies. However, the proximity of the tumor to important anatomical structures such as the orbit and skull base makes treatment difficult [1]. Cancers involving the anterior skull base were considered incurable; however, in 1963, Ketcham et al. forged new frontiers when they published a combined craniofacial resection series with good survival figures [2]. Today, surgeons can readily access the tumor domain. The indicated treatment with the best results is complete surgical resection of the tumor followed by reconstruction with postoperative radiotherapy. The biggest challenge of sinonasal reconstruction is to repair a complex three-dimensional structure with varying thickness of the tissue covering it, restore its function and aesthetic appearance to the greatest possible extent, and achieve facial symmetry with a good aesthetic outcome [3]. Herein, we report a case of reconstruction with an anterolateral thigh (ALT) free flap and galeal flap division for a huge skull defect following a rare sinonasal cancer resection.
A 51-year-old male patient with 7 pack years smoking history and with no specific medical or surgical history suddenly developed tenderness in the forehead for which he visited our hospital in June 2022. Preoperative magnetic resonance imaging (MRI) showed a malignant soft tissue mass involving the bilateral frontoethmoidal sinuses, with adjacent bony erosion. Extension to the overlying subcutaneous tissue of the nasal dorsum and suspicious focal dural involvement in the lateral aspect of the right lateral orbital gyrus were observed, and the patient was diagnosed with paranasal sinus cancer of stage T4bN0M0 (Fig. 1). Positron emission tomography-computed tomography revealed no evidence of metastasis. Surgical treatment was planned after a multidisciplinary discussion with the neurosurgery (NS) and otorhinolaryngology (ENT) teams.
The surgery was performed on August 26, 2022, in cooperation with the NS and ENT teams. First, the plastic surgery team preserved the superficial temporal artery and vein, and an incision was made along the bicoronal design. Subsequently, a bifrontal craniectomy involving the frontal sinus was performed by the NS team. Suspicious findings of periorbital and dural invasion around the right frontal sinus observed on preoperative MRI were to the extent that the tumor had disrupted the bone and was in contact with the surrounding structures. The dura in this area was excised and duroplasty was performed. In addition, the upper ethmoid bone infiltrated by the tumor was removed, and the parts that showed soft tissue infiltration through the anterior sinus wall near the glabella and the subcutaneous tissue and skin with the most severe infiltration were resected. The ENT team then performed a bilateral middle turbinectomy and removed the posterior ethmoid mucosa.
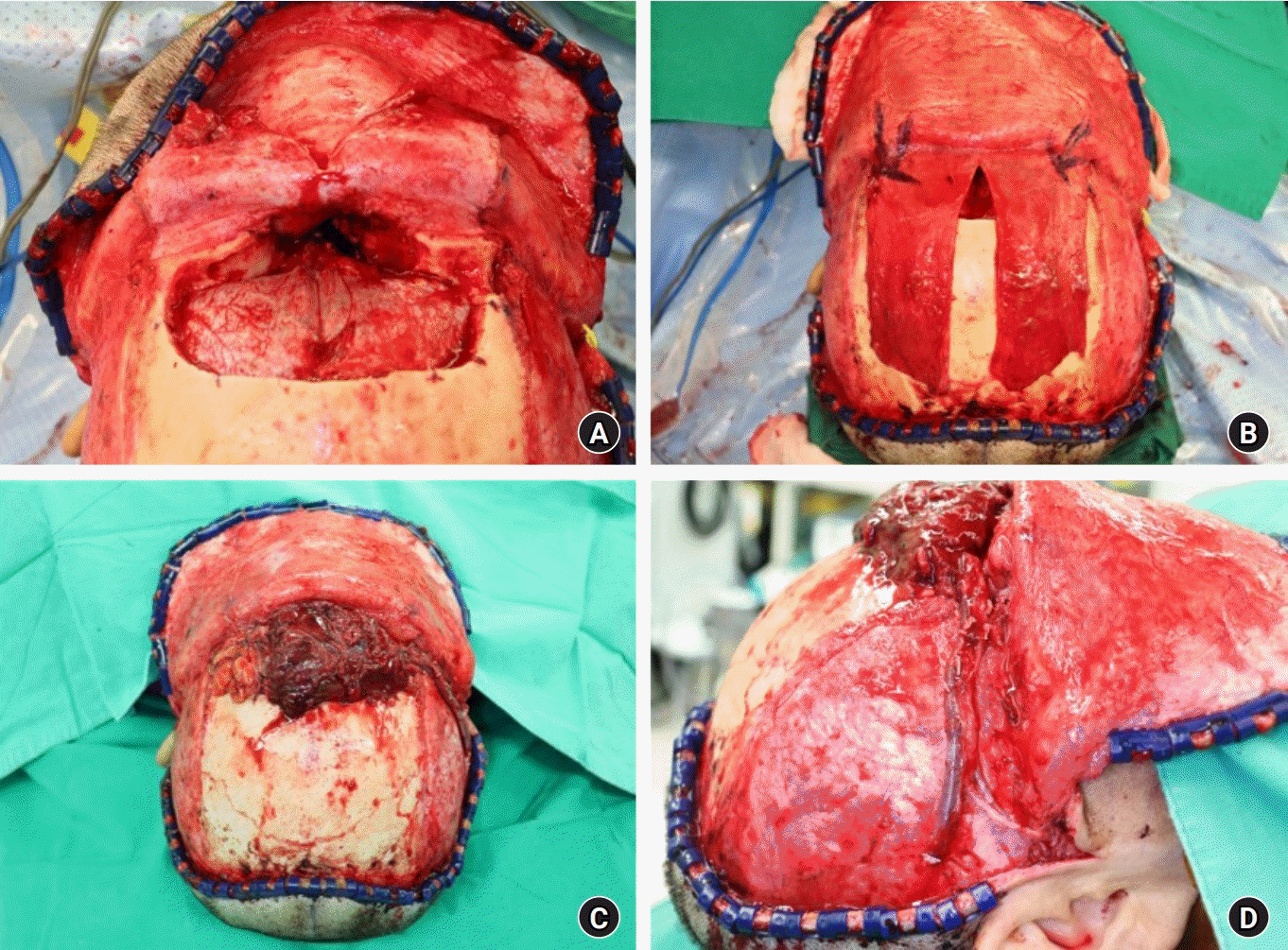
After resection of the tumor, an anterior skull base defect, which communicated between the intracranium and the nasal cavity, and a 3×3-cm skin defect at the glabella were noted (Fig. 2). The galeal flap was divided to create an anatomical and functional barrier to communication between the nasal cavity and intracranium. An ALT free flap was planned to cover the base of the anterior skull. The ALT free flap was harvested from the right thigh of the patient and fixed to the frontal bone. Sufficient volume was harvested, including the vastus lateralis muscle, in order to prevent the development of depressed forehead that can occur after reconstructing a large anterior skull defect. The flap measured 20×8 cm and the pedicle length was 7 cm. A microanastomosis was performed to connect one artery and two veins (donor: descending branch of the lateral circumflex femoral artery and venae comitantes/ recipient: left superficial temporal artery, venae comitantes, and occipital vein). Good circulation was confirmed after microanastomosis. Skin defects in the glabella were also covered with the harvested ALT free flap. The ALT flap was completely de-epithelialized except for the skin island paddle at the glabella defect site. One hemovac was applied to the anterior scalp to prevent hematoma formation, and a continuous irrigation system was applied from the right nostril through the nasal cavity to the pharynx.
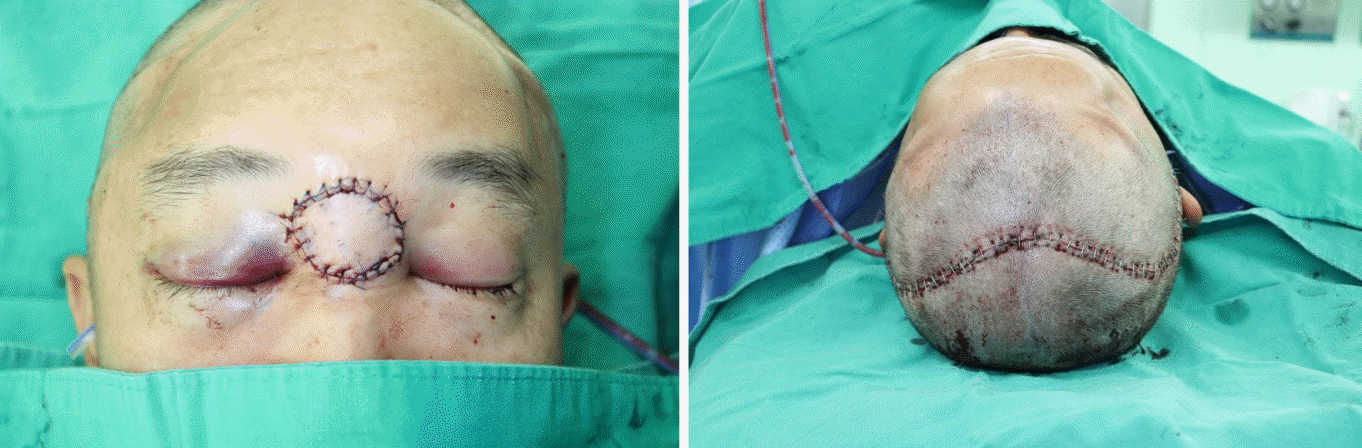
The surgery took approximately 13 hours, and there were no abnormalities in the blood flow of the reconstructed flap after the surgery. The flap was warm and soft, and Doppler sound was well traced (Fig. 3).
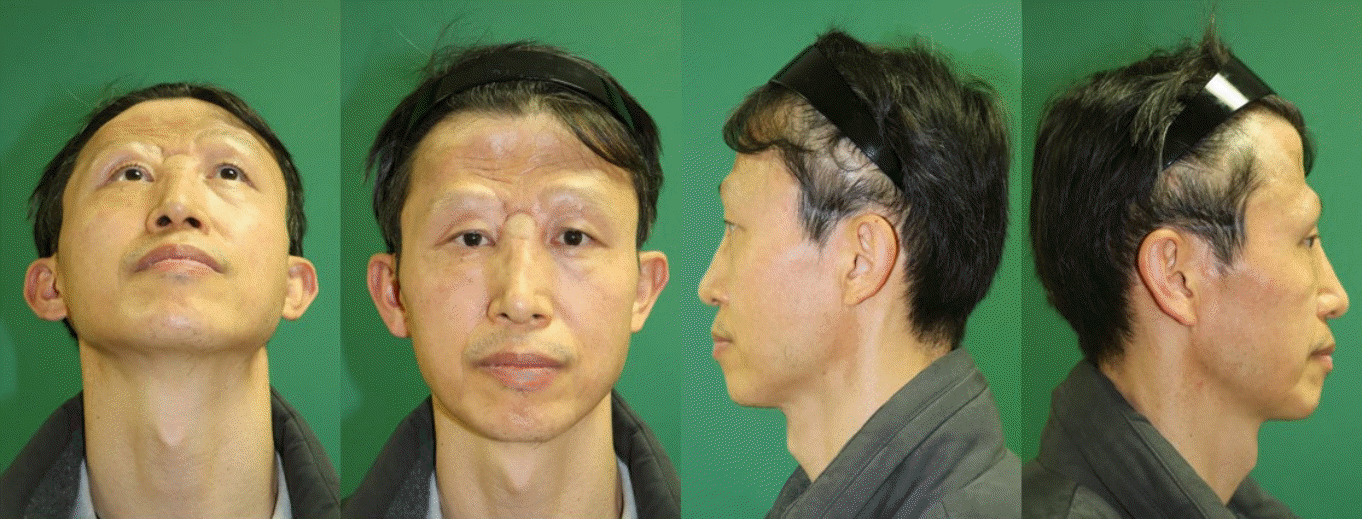
Postoperatively, there were no complications, such as cerebrospinal fluid (CSF) leakage, meningitis, tension pneumocephalus, wound infection, flap necrosis, or mucocele. Six weeks after the surgery, the patient received a total radiation dose of 6,000 cGy in 30 treatments, after which the flap remained intact without any subsequent complication (Fig. 4).
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Sinonasal tumors are a rare pathological entity, representing approximately 3% of upper respiratory tract tumors, characterized by marked anatomopathological diversity [1]. First described by Frierson et al. in 1986, sinonasal undifferentiated carcinoma is an uncommon aggressive malignancy of the nasal cavity and paranasal sinuses [3]. The affected areas include the nasal cavity and paranasal sinuses. In advanced cases, the tumors may extend to involve the surrounding anatomical structures, with no significant clinical symptoms until late in the course of the disease. Their proximity to vital structures such as the optic nerves and brain poses a challenge for surgeons when performing reconstructive treatment. The common extension areas for this type of tumor are the cribriform plate, crista galli, roof of the ethmoid, orbit, and occasionally, facial soft tissue. Extensive tumor resection with clear margins may result in sizeable cranial and facial skin defects that must be covered with the aid of a multidisciplinary team [1]. Therefore, an interdisciplinary approach including ENT-surgeons, neurosurgeons, and eventually maxillofacial and plastic surgeons is inevitable, since these disciplines combine diverse operative strategies, comprising highly developed microsurgical, endoscopic, and reconstructive techniques.
The indicated treatment with the best results is complete surgical resection of the tumor, followed by reconstruction and postoperative radiotherapy. Tumor resection may create extensive skull base defects and produce a free conduit between the paranasal sinuses and intracranial space when tumors arising in the anterior skull base invade both its soft and hard tissues. Following tumor extirpation, skull and cranial base defects require precise and durable reconstruction to form a watertight dural seal, providing a barrier between the contaminated sinonasal space and sterile subdural compartment, preventing airflow into the intracranial space, and maintaining a functional sinonasal system [4].
The major goal of reconstruction is to achieve a watertight dural seal to reestablish the separation of the intracranial contents from the extracranial oral and nasal cavities that are deemed contaminated [5,6]. Inadequate reconstruction may result in postoperative CSF leaks that may lead to serious complications such as infection, CSF fistula formation, meningitis, and tension pneumocephalus. CSF leaks have been reported to occur in 2% to 13% of skull base surgeries. Associated meningitis has been observed in 1% to 5% of cases; intracranial abscesses, tension pneumocephalus, and osteomyelitis occur rarely [5].
After the resection of craniofacial tumors, it is essential to isolate the cranial cavity, with some vascularized tissues, from the extracranial space to prevent intracranial infections in the brain. Important factors must be considered when selecting reconstruction options for the anterior skull base [6,7]. The primary goal for skull base reconstruction includes creating a durable watertight separation between the intradural contents and external exposure. This is due to the high risk of complications from persistent CSF fistulas, such as in meningitis and pneumocephalus, and the associated mortality that increases over time. We therefore used the galeal flap division to create an anatomical and functional barrier, preventing communication between the nasal cavity and intracranial cavity. Galeal flap division played its role as an effective watertight barrier and no complication including CSF leakage occurred postoperatively. Secondary goals involve the closure of dead space, return of function, and the restoration of the cosmesis. The location and volume of the defect are perhaps the most important factors, as these factors determine the extent of communication between the intradural and extradural spaces, as well as which tissue options are appropriate for reconstruction [7]. By harvesting enough volume during the ALT free flap, we were able to create a rounded forehead with no depression, and the patient was also very satisfied with the results. Another crucial factor that can affect the reconstructive strategy is patient history. For those with a history of diabetes and tobacco use, there is a higher risk of skin necrosis, which may require adaptation of this approach. Additional host factors that should be assessed include history of radiotherapy, previous surgery, availability of local reconstructive tissues, and previous reconstruction attempts [1,7]. Surgeons should consider all these factors when deciding how to proceed with reconstruction.
Following skull base resection, free flaps can be used to treat massive defects with excellent surgical results and low complication rates [4]. Microvascular free tissue transfer has broadened the field of skull base extirpation and is now the mainstay of the reconstruction of complex three-dimensional anterior skull base defects. This technique provides adequate tissue bulk with a rich and reliable blood supply, allowing for improved healing, decreased hospitalization, and decreased complications, including CSF leak and meningitis. Commonly used soft tissue free flaps for anterior skull base defects include the rectus abdominis, latissimus dorsi, and ALT because of their large skin paddles and adequate amount of muscle bulk [8]. The ALT flap has been described as a reasonable alternative to the rectus abdominis flap, with its blood supply from the descending branch of the lateral femoral circumflex artery and relatively low donor-site morbidity. Its advantages include a two-team approach, minimal donor-site morbidity, volume control when minimal muscle is harvested, and flap reliability [9,10]. Moyer et al. [9] described their experience with the free vastus lateralis flap in seven patients with cranial base defects treated between 1997 and 2001. During a mean follow-up of 10 months, no CSF leaks or meningitis episodes were reported. Interestingly, the authors noted that the fascia lata could also be harvested along with the vastus lateralis to achieve a vascularized layer for dural repair. Moreover, Wang et al. [10] in their study described 34 patients who underwent ALT with an average flap size 138.7 cm2 for skull base reconstruction with a 29% complication rate and no flap loss. The authors advocate the flap as robust and easy for a two-team approach, given the distance from the head and the ability to simultaneously harvest a saphenous vein for length and to be used as a cable-grafting facial nerve if needed. Wang et al. [10] reported 12 patients with lateral skull base defects without flap loss.
For malignant anterior skull base tumors, postoperative radiation is anticipated, which can predispose patients to delayed flap necrosis and secondary CSF leakage [6]. However, no complications of CSF leakage, meningitis, or tension pneumocephalus developed in our patient. We successfully reconstructed a large defect in the anterior skull base that included a skin defect in the glabella area, using a precisely designed ALT free flap and galeal flap division. Postoperatively, despite the extensive radiotherapy following the operation, the patient experienced no subsequent complication. Based on this experience, other complex and difficult reconstructions of the head and neck should be successfully performed with an appropriate free flap. In conclusion, management of large craniofacial defects after advanced tumor resection is an important challenge for most surgeons. Free tissue transfer can provide reliable, vascularized tissue to fill volume defects created by skull base resections and reinforce dural closures to prevent CSF leaks and meningitis. To achieve safe and satisfactory results, it is important to adapt the technique to the particularities of each patient while preserving vital functions. However, these challenges allow us to implement improved reconstructive techniques and methods, striving to preserve patients’ quality of life to the greatest extent possible.
References
1. Palade DO, Hainarosie R, Pertea M, et al. Reconstructive challenges of sinonasal tumors: a case report. Exp Ther Med. 2022; 23:419.

2. Bridger GP, Kwok B, Baldwin M, Williams JR, Smee RI. Craniofacial resection for paranasal sinus cancers. Head Neck. 2000; 22:772–80.

3. Gorelick J, Ross D, Marentette L, Blaivas M. Sinonasal undifferentiated carcinoma: case series and review of the literature. Neurosurgery. 2000; 47:750–5.

4. Gil Z, Abergel A, Leider-Trejo L, et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007; 17:25–37.

5. Yano T, Tanaka K, Kishimoto S, Iida H, Okazaki M. Reliability of and indications for pericranial flaps in anterior skull base reconstruction. J Craniofac Surg. 2011; 22:482–5.

6. Eloy JA, Choudhry OJ, Christiano LD, Ajibade DV, Liu JK. Double flap technique for reconstruction of anterior skull base defects after craniofacial tumor resection: technical note. Int Forum Allergy Rhinol. 2013; 3:425–30.

7. Nameki H, Kato T, Nameki I, Ajimi Y. Selective reconstructive options for the anterior skull base. Int J Clin Oncol. 2005; 10:223–8.

8. Ein L, Sargi Z, Nicolli EA. Update on anterior skull base reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2019; 27:426–30.

Fig. 1.
Preoperative magnetic resonance imagings (MRIs). A malignant soft tissue mass involving the bilateral fronto-ethmoidal sinuses, with adjacent bony erosion, was proven to be paranasal sinus cancer. It extended to the overlying subcutaneous tissue of the dorsum of the nose. (A) Coronal view of the patient's MRI. (B) Sagittal view of the patient's MRI. (C) Axial view of the patient's MRI. (D) Axial view of the patient's MRI. Suspicious focal dural involvement was noted in the lateral aspect of the right lateral orbital gyrus (arrow).
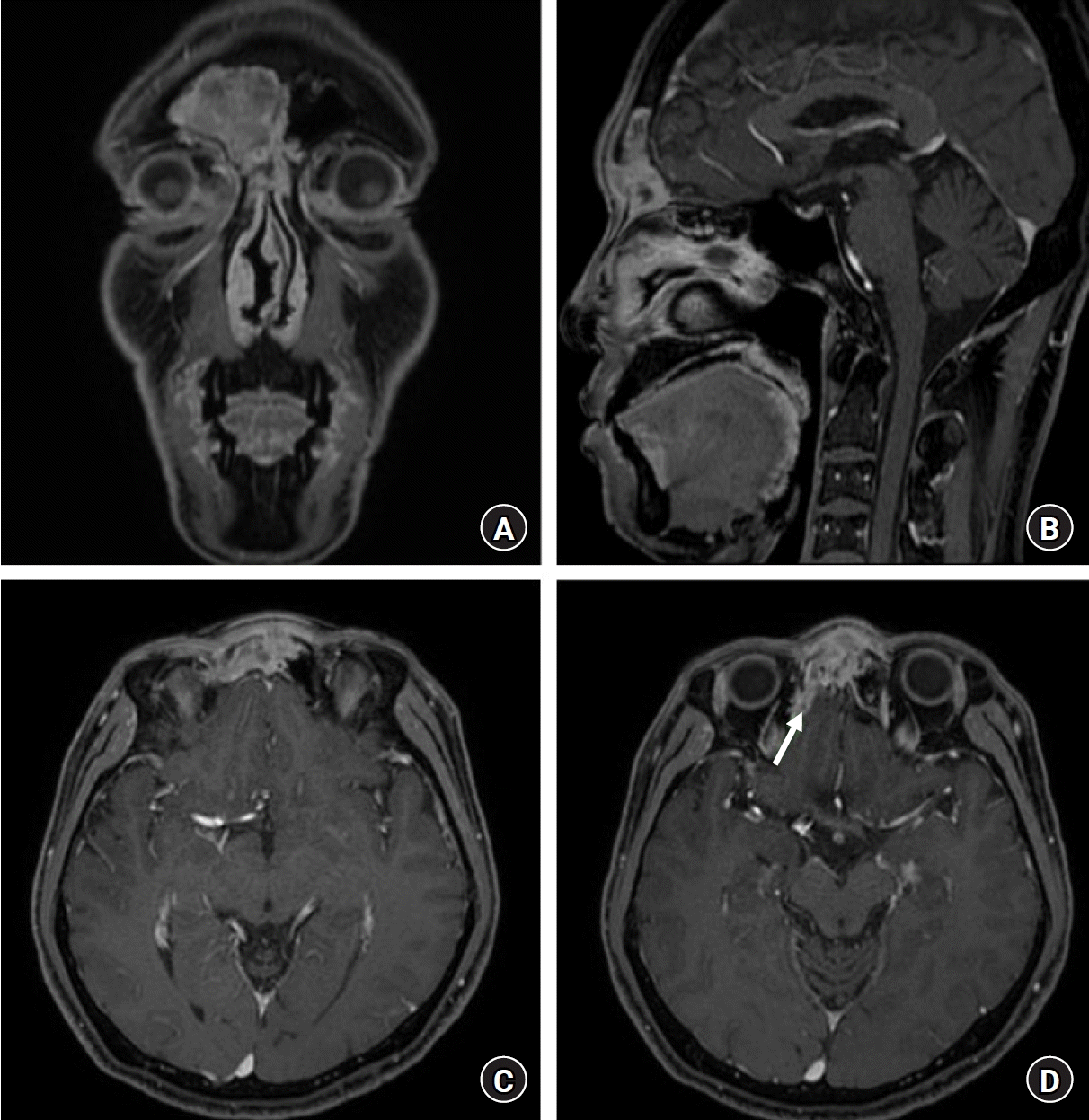
Fig. 2.
Photographs of the intraoperative procedure. (A) The defect of the anterior skull base after resection of the tumor, with communication between the intracranial cavity and the nasal cavity. (B) The divided galeal flap, which was designed to create an anatomical and functional barrier to communication between the intracranial cavity and the nasal cavity. (C) Photograph of the harvested anterolateral thigh free flap covering the frontal skull base defect. (D) Photograph of the microanastomosis performed. (donor: descending branch of the lateral circumflex femoral artery and venae comitantes; recipient: left superficial temporal artery, venae comitantes, and occipital vein).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download