Abstract
Background
Pyruvate dehydrogenase complex (PDHC) deficiency is a rare mitochondrial disorder caused by a genetic mutation affecting the activity of the PDHC enzyme, which plays a major role in the tricarboxylic cycle. Few cases of surgery or anesthesia have been reported. Moreover, there is no recommended anesthetic method.
Case
A 24-month-old child with a PDHC deficiency presented to the emergency room with respiratory failure, mental decline, systemic cyanosis, and lactic acidosis. During hospitalization period, the patient presented with pneumothorax, pneumoperitoneum, and multiple air pockets in the heart. Two surgeries were performed under general anesthesia using an inhalational anesthetic agent. The patient was discharged with home ventilation.
Go to : 
Pyruvate dehydrogenase complex (PDHC) deficiency is a rare mitochondrial disorder that primarily affects the brain and results in decreased adenosine triphosphate (ATP) production and energy deficiency [1]. It is caused by a genetic mutation that affects the activity of the PDHC enzyme, which plays a major role in the tricarboxylic acid (TCA) cycle. PDHC deficiency can lead to a variety of symptoms, including intrauterine growth retardation, developmental delay, hypotonia, intermittent ataxia, lactic acidosis, facial dysmorphism, and cerebral atrophy [2]. The exact prevalence of PDHC deficiency remains unknown owing to its rarity [3]. To the best of our knowledge, few cases related to surgery or anesthesia have been reported. This makes it challenging to determine the best anesthetic method for patients with PDHC deficiency. Therefore, further research and case reports are required to establish appropriate anesthesia protocols and improve the perioperative outcomes of patients with PDHC deficiency. Anesthesiologists need to raise awareness about PDHC deficiency and develop safe and effective anesthetic plans. In this context, we present the anesthetic management of a 24-month-old male patient with PDHC deficiency who underwent two consecutive surgeries under general anesthesia. We aimed to provide insights into the perioperative considerations in the management of patients with PDHC deficiency patients undergoing surgery and anesthesia.
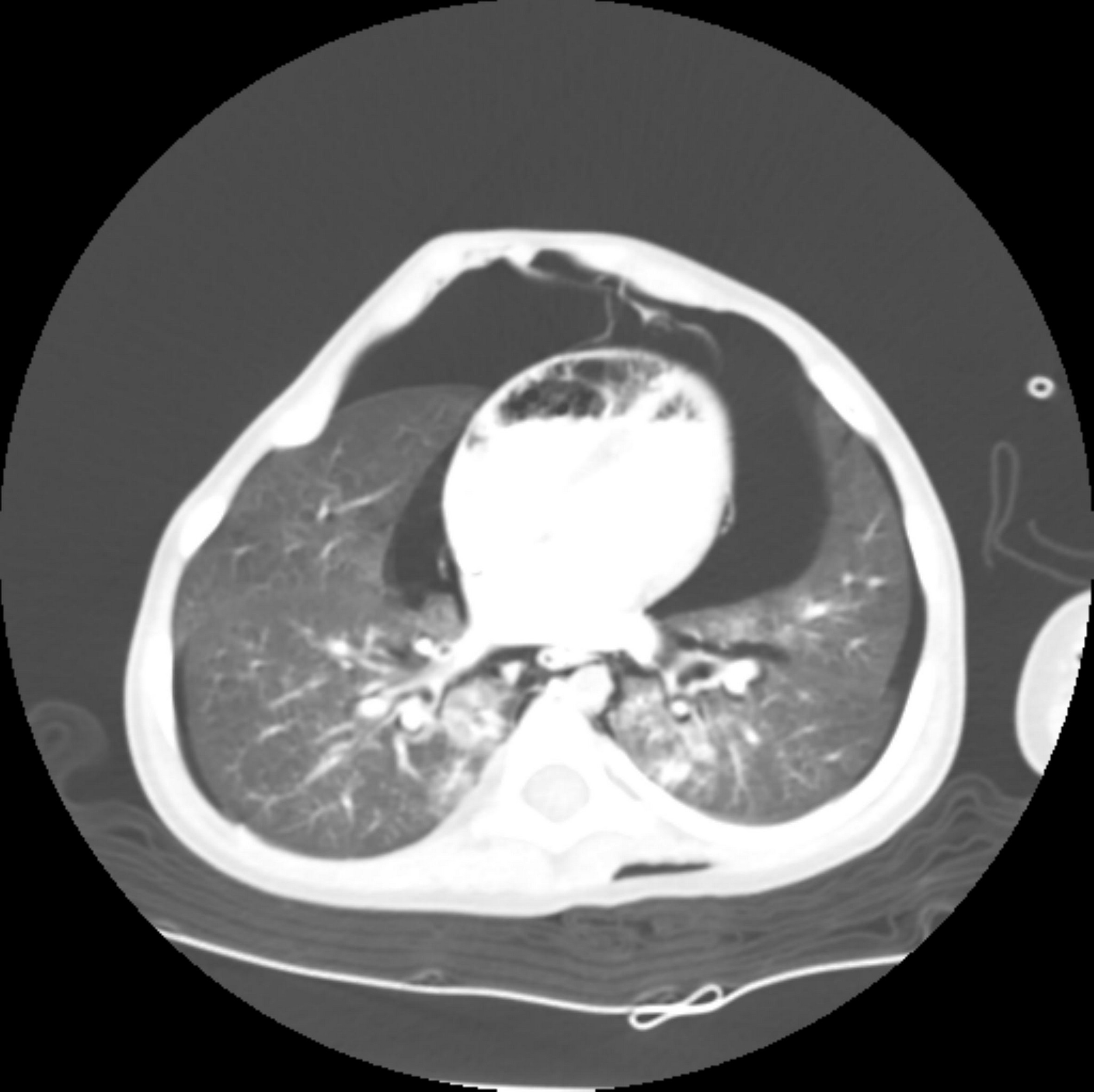
The current report was approved by the Institutional Review Board of our hospital, and the requirement for informed consent was waived (IRB no. 2303-013-125). A 24-month-old male patient with a height of 87 cm and a weight of 10.5 kg presented to the emergency room due to respiratory failure, mental decrease to a stupor state, systemic cyanosis, and lactic acid increase up to 12.3 mM/L (normal range: 0.7–2.5 mM/L). Initial arterial blood gas analysis result was pH 6.80, PaCO2 131 mmHg, PaO2 75 mmHg. The child was previously diagnosed with pyruvate dehydrogenase E1-alpha deficiency two months after birth. At the time of diagnosis, the child’s laboratory result showed lactic acid 6.4 mM/L. There were no abnormal findings in the parental phenotype, and the parental genetic test results were normal. Therefore, the patient had a de novo pathogenic variant of pyruvate dehydrogenase E1-alpha deficiency. He had a history of multiple admissions and discharges with similar events. The main clinical features of the child were lactic acidosis, mental decline, respiratory failure, and systemic cyanosis. The child received mechanical ventilation and bicarbonate administration after hospitalization. During this hospitalization period, he presented with abdominal distension and ileus, and aggravation and remission continued repeatedly. Computed tomography (CT) scan was conducted on the 30th day of hospitalization to determine the cause. CT scan revealed a pneumothorax, pneumomediastinum, pneumoperitoneum, and multiple air pockets in the heart, retroperitoneum, extraperitoneal space, scrotum, and renal vessels (Fig. 1). It was assumed that the ventilator-induced lung injury occurred due to the long period of mechanical ventilation, resulting in these conditions. There were no other events, such as an accidental air injection.
Bilateral chest tube insertion was performed before surgery and loop ileostomy was planned for an emergency surgery. The patient arrived in the operating room in an intubated state with an endotracheal tube (Inner diameter 4.5 mm). Anesthesia was induced with 2.0 vol% sevoflurane and 10 mg rocuronium. Invasive blood pressure monitoring was performed via arterial cannulation of the left radial artery. Capnography, electrocardiography, pulse oximetry, core temperature, anesthetic agent concentration, and airway pressure was also monitored. Neuromuscular monitoring was excluded because the appropriate monitoring size was unavailable. A circulating-water mattress was used to maintain the patient’s body temperature. Maintenance of anesthesia was performed with 2.0 vol% sevoflurane, 0.02–0.03 mg/h of intravenous remifentanil infusion. Mechanical ventilation was set at a tidal volume of 60 ml, respiratory rate 24 per min, and FiO2 0.74 (2.0 L/min oxygen, 1.0 L/min air). Oxygen saturation was maintained at 95% and end-tidal CO2 at 32 mmHg. Balanced crystalloid solution was administered as an intravenous fluid. The vital signs of the patient maintained stable throughout the surgery. The duration of the operation was 1 h and the anesthesia lasted for 1 h and 35 min. The patient was transferred to the surgical intensive care unit while maintaining intubation. Preoperative venous blood gas analysis and postoperative arterial blood gas analysis data are presented in Table 1.
For the first week after the surgery, hyperbaric oxygen therapy with FiO2 1.0 was conducted to resolve air pockets in the heart. After terminating the hyperbaric oxygen therapy, the patient’s ventilation was supported by the synchronized intermittent mandatory ventilation and continuous positive airway pressure modes. Two weeks after the first operation, a second operation was conducted as an elective surgery for tracheostomy, ileostomy take-down, feeding gastrostomy and fundoplication. The patient intubated with an endotracheal tube (Inner diameter 4.5 mm) arrived in the operating room and anesthesia was induced using 2.0 vol% sevoflurane and 8 mg rocuronium. Capnography, electrocardiography, pulse oximetry, core temperature, anesthetic agent concentration, and airway pressure were monitored. Neuromuscular monitoring was not performed because of its unavailability. A circulating-water mattress and a Bair Hugger™ (3M Inc.) were used to maintain the body temperature of the patient. Maintenance of anesthesia was performed with 2.0 vol% sevoflurane, and intravenous fluid using 5% dextrose water. Mechanical ventilation was set at a tidal volume of 60 ml, respiratory rate 28 per min, and FiO2 0.37–0.44 (0.3–0.5 L/min oxygen, 1.2 L/min air). The oxygen saturation was maintained at 96–100% and end-tidal CO2 at 35 mmHg. Due to the blood loss during the surgery, one packed red blood cell was also administered by a different intravenous route. Dopamine infusion was used during the surgery to assist hemodynamics. After administering dopamine infusion, the patient’s vital signs maintained stable. The surgery lasted for 4 h and 50 min and the total anesthesia time was 5 h and 35 min. The patient was transferred to the surgical intensive care unit with a tracheal cannula fixed at the tracheostomy site. The preoperative and postoperative arterial blood gas analysis data for the second operation are also presented in Table 1. Looking through the patient’s two surgeries, lactic acidosis was apparent in the acute stage.
The patient received ventilator care in the surgical intensive care unit for three days and was switched to high-flow tracheal oxygenation. No events such as desaturation, tachypnea, and dyspnea were observed. The patient was transferred to the general ward on the ninth postoperative day. After the preparation of home ventilation to support the patient’s breathing after discharge, the patient was finally discharged on the 14th postoperative day.
Following 14 months after surgery, the patient visited the emergency room four times. He continued to present with symptoms such as lactic acidosis and mental decline, and was repeatedly hospitalized with a diagnosis of pneumonia. After several days of conservative treatment, including mechanical ventilation and bicarbonate administration, the patient’s symptoms were relieved and he was discharged from the hospital. However, he was repeatedly readmitted until now. Moreover, the dependence of the patient on home ventilation continues to increase so far.
Go to : 
PDHC is a complex of five components that converts pyruvate into acetyl-CoA, which then enters the TCA cycle for ATP production. The five components are E1, an α-keto acid decarboxylase; E2, a dihydrolipoyl transacylase; E3, a dihydrolipoyl dehydrogenase; protein X, an extra lipoate-containing protein; and pyruvate dehydrogenase phosphatase [1]. E1 enzyme contains subunits, which are divided into alpha and beta subunits. If any of these components are missing, the conversion of pyruvate to acetyl-CoA cannot proceed, resulting in the accumulation of pyruvate and lactate [4], leading to symptoms such as lactic acidemia and central nervous system abnormalities. The most common type is pyruvate dehydrogenase E1-alpha deficiency, which accounts for 76–85% of PDHC deficiencies. Approximately 60–63% of pyruvate dehydrogenase E1-alpha deficiencies are de novo pathogenic variants and the rest are exhibited in X-linked inheritance [2].
The presentation of this disease varies depending on the onset age. There are three main categories: neonatal onset, infantile onset, and later childhood onset. The neonatal form has the most severe enzyme deficiency and presents with lethal lactic acidosis and structural brain anomalies such as agenesis of the corpus callosum. Patients with infantile onset may present with chronic lactic acidosis, psychomotor retardation, and cystic lesions in the brainstem and in the basal ganglia. If the disease appears in late childhood, patients have less impaired enzyme activity and may present intermittent ataxia, paroxysmal dystonia, paroxysmal dyskinesia, and less acidosis [1]. Lactic acidosis can be aggravated by carbohydrate consumption. Therefore, implementing a ketogenic diet is recommended. This dietary approach can help to reduce lactate levels, but its long-term efficacy remains unclear [1]. Thiamine, a co-factor of pyruvate dehydrogenase, is also used routinely but only a minority show response to thiamine. Patients with pyruvate dehydrogenase E1-alpha deficiency exhibit the most favorable response to thiamine [2].
The patient in this case was a 24-month-old male patient with the most common pyruvate dehydrogenase E1-alpha deficiency. The child can be classified as having infantile onset. The child exhibited chronic lactic acidosis and psychomotor retardation. However, no structural brain anomalies or neurologic deficits were observed on the brain MRI. His daily diet was based on formula milk and enteral nutrition formulas. Therefore, he was not strictly on a ketogenic diet. He was treated with the maximum dose of thiamine, which is 1 g/day.
PDHC deficiency is a type of mitochondrial disorder. When administering anesthesia to patients with mitochondrial disorders, complications such as prolonged muscle relaxation effects [5], acute respiratory failure after anesthesia recovery [6], malignant hyperthermia [7], and propofol infusion syndrome [8] can be observed. Although, in our patient, there were no signs of such adverse events and no abrupt abnormal changes in electrocardiography, capnography, body temperature, and blood pressure, anesthesiologists should always consider multiple factors when administering anesthesia to patients with PDHC deficiency.
First, airway problems can be caused by facial dysmorphism, which can be observed in patients with PDHC deficiency. Such facial features include a flat wide nasal bridge, long philtrum, thin upper lip, low-set ears, and a high arched palate [3,9]. Intubation can be difficult due to facial dysmorphism [10]. Therefore, additional methods such as using a video laryngoscope can be considered. In this case, he showed a wide nasal bridge and flared nostrils. However, no other facial deformities were observed. Thus, no airway problems were identified.
Second, close attention should be paid to the acid-base imbalance. Precipitating factors that trigger an increase in lactate levels should be avoided. Sepsis, hypothermia, hypoxemia, hypocarbia, and decreased cardiac output all contribute to lactate increase [4,11]. Anesthetic management should be performed carefully to avoid these events and make stress-free situations. If possible, arterial blood gas analysis should be performed at regular intervals to closely monitor lactic acidosis.
The selection of intraoperative intravenous fluids also needs to be carefully considered. Lactate containing intravenous solutions should not be administered as the lactate load only increases [12]. In this case, we used a balanced crystalloid solution that did not contain lactate during the first surgery, and we used 5% dextrose water for the second surgery. Both fluids were helpful as they did not contribute to an increase in lactate levels. Furthermore, owing to a decent amount of estimated blood loss during the second surgery, we used one packed red blood cell for transfusion and no side effects were observed.
Third, the choice of drugs for anesthesia induction and maintenance is influenced by the inhibitory effect of the drug-induced suppression of gluconeogenesis, which can exacerbate coexisting metabolic acidosis [13]. To the best of our knowledge, no recommendations are currently available for which anesthetic agents should be used. In this case, anesthesia was maintained with sevoflurane and no complications were observed. According to previous literature, successful cases using sevoflurane have been observed [12,13]. Also, there have been two successful cases of total intravenous anesthesia using propofol and fentanyl [10], and midazolam and remifentanil [11]. Based on the reported cases so far, we can confirm that either method can be utilized with a close monitoring. However, anesthesiologists should be aware of the possibility of malignant hyperthermia when using volatile agents. On the other hand, the possibility of propofol infusion syndrome should be considered when propofol is used as the primary anesthetic agent. Proper monitoring such as capnography, electrocardiography, and core temperature monitoring should be applied to detect early signs of these events.
Since there was no appropriate size of neuromuscular monitoring for this patient in our hospital, it was not possible to check the neuromuscular transmission status of the patient. However, we had to use a neuromuscular blocking agent more frequently because of multiple episodes of spontaneous respiration. Based on this finding, it is likely that there was no prolongation of the muscle relaxation effect.
Through this case and literature review, we confirmed that anesthesiologists should consider the multiple factors mentioned above when anesthetizing a patient with PDHC deficiency. Moreover, monitoring lactic acid levels might be a helpful indicator to predict the clinical course of a patient with PDHC deficiency during the perioperative period.
Go to : 
Notes
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Won Yong Lim, Hyeon-Jeong Lee. Data curation: Won Yong Lim, Hyeon-Jeong Lee, Eun Ji Park, Soeun Jeon.
Project administration: Hyeon-Jeong Lee. Visualization: Hyeon-Jeong Lee, Wangseok Do, Dowon Lee.
Writing - original draft: Won Yong Lim.
Writing - review & editing: Won Yong Lim, Hyeon-Jeong Lee, Wangseok Do, Hyae Jin Kim.
Resources: Eun Ji Park.
Supervision: Hyeon-Jeong Lee, Jeong-Min Hong.
Go to : 
REFERENCES
1. Kliegman R, St. Geme JW, Blum NJ, Shah SS, Tasker RC, Wilson KM, et al. Nelson textbook of pediatrics. 21st ed. Philadelphia: Elsevier;2020. p. 3487–9.
2. Ganetzky R, McCormick EM, Falk MJ. Primary pyruvate dehydrogenase complex deficiency overview. GeneReviews® [Internet]. 1993. [updated 2021 Jun 17; cited 2023 Mar 14]. Available from https://www.ncbi.nlm.nih.gov/books/NBK571223/.
3. Patel KP, O'Brien TW, Subramony SH, Shuster J, Stacpoole PW. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metab. 2012; 106:385–94.

4. Dierdorf SF, McNiece WL. Anaesthesia and pyruvate dehydrogenase deficiency. Can Anaesth Soc J. 1983; 30:413–6.

5. Finsterer J, Stratil U, Bittner R, Sporn P. Increased sensitivity to rocuronium and atracurium in mitochondrial myopathy. Can J Anaesth. 1998; 45:781–4.

6. Grattan-Smith PJ, Shield LK, Hopkins IJ, Collins KJ. Acute respiratory failure precipitated by general anesthesia in Leigh’s syndrome. J Child Neurol. 1990; 5:137–41.

7. Fricker RM, Raffelsberger T, Rauch-Shorny S, Finsterer J, Muller-Reible C, Gilly H, et al. Positive malignant hyperthermia susceptibility in vitro test in a patient with mitochondrial myopathy and myoadenylate deaminase deficiency. Anesthesiology. 2002; 97:1635–7.

9. De Meirleir L, Lissens W, Denis R, Wayenberg JL, Michotte A, Brucher JM, et al. Pyruvate dehydrogenase deficiency: clinical and biochemical diagnosis. Pediatr Neurol. 1993; 9:216–20.

10. Milojevic I, Simic D. Anesthesia in pyruvate dehydrogenase deficiency. Paediatr Anaesth. 2008; 18:794–5.

11. Ro J, Kim EJ, Lee JH, Lee SG, Ban JS, Min BW. Anesthetic experience in a pediatric patient with pyruvate dehydrogenase complex (PDHC) deficiency: a case report. Korean J Anesthesiol. 2008; 55:629–33.

12. Gilmore DA, Mayhew J. Anesthesia in a child with pyruvate dehydrogenase deficiency: a case report. AANA J. 2008; 76:432–3.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download